Itrim.de
FROM THE DEPARTMENT OF MEDICINE Karolinska Institutet, Stockholm, Sweden VERY LOW ENERGY DIETS IN THETREATMENT OF OBESITY
Studies of Obstructive Sleep Apnoea, Side-Effects, and Treatment Discontinuation Al previously published papers were reproduced with permission from the publisher. Published by Karolinska Institutet. Printed by E-print AB. Kari Johansson, 2012 ISBN 978-91-7457-736-5 Choose a job you love and you will never have to work a day in your life. ABSTRACT
Background The prevalence of obesity has increased dramatical y during the last decades worldwide.
Obesity is associated with increased risk of morbidity and mortality, leading to an increased suffering
for the individual patient and an increased burden on the health care system. Currently, the most
effective treatment is bariatric surgery. Since bariatric surgery cannot be provided to al obese patients,
other non-surgical obesity treatment methods are needed.
Aim The overal objective of this thesis was to evaluate effects and side-effects of very low energy
diets (VLEDs), as wel as to characterise treatment discontinuation. Specific objectives were to evaluate
weight loss as treatment option for patients with obstructive sleep apnoea (OSA; Study I&II); to assess
the risk of gallstones requiring hospital care, and cholecystectomy, in a commercial weight loss
programme using VLED or low energy diet (LED; Study I I); and to characterise discontinuation
patterns in obesity treatment programmes by analysing data from anti-obesity drug trials (Study IV).
Methods The study on OSA and weight loss (Study I&II) consisted of a randomised control ed trial
(RCT) fol owed by an observational fol ow-up for a total duration of one year. Included were obese
men (n=63, BMI 30-40, aged 30-65 years) with moderate to severe OSA (apnoea-hypopnoea index
(AHI) ≥15) treated with CPAP. The intervention consisted of a hospital-based weight loss programme,
using VLED (554 kcal/day) to promote weight loss for nine weeks After the RCT was finished the
controls also received VLED. The VLED, in both groups, was fol owed by a 43-week weight loss
maintenance phase. Study I I was a one-year matched cohort study of consecutively enrol ed adults in a
commercial weight loss programme in Sweden between 2006 and 2009 (n=6,640; mean age 46y; 83%
women; mean BMI 33). The intervention included a three-month weight loss phase, consisting of either
VLED (500 kcal/day) or LED (1,200-1,500 kcal/day), fol owed by a nine-month weight loss maintenance
phase. Gallstones requiring hospital care and cholecystectomies during the one-year programme were
col ected from the National Patient Register. Study IV was a systematic review and meta-analysis
including published placebo-control ed anti-obesity trials of orlistat, sibutramine and rimonabant
(n=13,457).
Results Study I&I : After the nine-week RCT the intervention group's mean body weight was 20 kg
lower than that of the control group, and its mean AHI was 23 events/h lower. In total 70% (44/63)
completed the one-year pooled observational fol ow-up. The AHI changes after nine weeks of VLED
(-58%) were largely maintained at one-year (-47%) fol owing the initial weight loss of 18 kg, and 12 kg
at one year. Study I I: The absolute risks of gallstones requiring hospital care and cholecystectomy were
found to be low, but three times higher in the VLED than the LED programme (hazard ratio 3.4 and
3.1, respectively; both P<0.001). While the risks were greater in the VLED compared to LED group,
the benefits in terms of one-year weight loss was also greater (11 vs 8 kg; P<0.001), and the
proportion remaining in the programme (82% vs 78%; P<0.001). Study IV: The overal combined one-
year dropout rates were high in both the drug (30-39%) and placebo arms (37%) of placebo-control ed
anti-obesity drug trials, but marginal y lower in the drug arms (pooled risk ratio 0.9; P=0.001).
Conclusion VLED-induced weight loss resulted in a significant reduction of moderate to severe OSA,
with the majority of the initial improvement maintained at one year. Albeit low, the risks of gallstones
and cholecystectomy were greater with VLED than LED treatment, as was weight loss. Treatment
discontinuation was lower both in the hospital-based weight loss programme and in the commercial
weight loss programme, as compared to pooled data from the placebo arms in anti-obesity drug trials.
LIST OF PUBLICATIONS
I
Johansson K, Neovius M, Trol e Lagerros Y, Harlid R, Rössner S, Granath F, Hemmingsson
E. Effect of a very low energy diet on moderate and severe obstructive sleep apnoea in obese
men: a randomised controlled trial. BMJ 2009 Dec 3;339:b4609.
Johansson K, Hemmingsson E, Harlid R, Trolle Lagerros Y, Granath F, Rössner
S, Neovius M.BMJ 2011 Jun 1;342:d3017.
Johansson K, Sundström J, Marcus C, Hemmingsson E, Neovius M. Risk of gal stones in a
commercial weight loss program using very low energy diet or low energy diet: matched
cohort study. Manuscript.
Johansson K, Neovius K, DeSantis SM, Rössner S, Neovius M. Obes Rev. 2009 Sep;10(5):564-75.
LIST OF ABBREVIATIONS
95%CI
95% confidence interval Action for Health in Diabetes Apnoea-hypopnoea index Alanine aminotransferase Baseline observation carried forward Cognitive behavioural treatment Continuous positive airway pressure Food and drug administration in the US Good clinical practice Glucagon-like peptide-1 Glycated hemoglobin High density lipoprotein International classification of diseases Intention-to-treat Low calorie diet Low density lipoprotein Last observation carried forward National Health and Nutrition Examination Survey National Weight Control Registry Oxygen desaturation >4% per hour of sleep Obstructive sleep apnoea Obstructive sleep apnoea syndrome Randomised control ed trial Respiratory disturbance index Sibutramine Cardiovascular Outcomes Trial Swedish Obese Subjects Thyroid stimulating hormone Very low calorie diet Very low energy diet World Health Organisation CONTENTS
1 INTRODUCTION
1.1 OBESITY AND OVERWEIGHT
1.1.1 Definition
Obesity and overweight are defined as abnormal or excessive fat accumulation that may impair health,
and classified, according to the World Health Organisation (WHO), by use of the body mass index
(BMI; kg/m2).1 A BMI ≥30.0 is classified as obesity, while a BMI between 25.0 and 29.9 is classified as
overweight, sometimes referred to as pre-obesity (Table 1).1
Table 1 Classification of adult overweight and obesity1
Classification
BMI (kg/m2)
Underweight
<18.5
18.5-24.9
Overweight
25.0-29.9
Obesity class I Obesity class II Obesity class III
Although BMI is used as a proxy for adiposity, it does not distinguish between fat and fat free mass, nor
does it take fat distribution into account. Other more specific measures of body fat include
measurement of body composition, such as dual energy X-ray absorptiometry, magnetic resonance
imaging and bioelectrical impedance analysis. However, the costs, the limited availability, or the
inaccuracy of some of these techniques limit their usefulness especial y on the population level. BMI is
therefore considered to provide a simple but useful measure of obesity.1
Waist circumference is commonly used as a surrogate measure for abdominal obesity (Table 2).
Visceral fat has been described to be more metabolically active than fat in general, and abdominal
obesity may therefore be associated with greater health risks than general obesity.1 Studies have
shown that waist circumference independently contributes to mortality risk beyond BMI.2
Table 2 Waist circumference thresholds
for a bdominal obesity in Caucasians3 Increased risk
High risk
1.1.2 Prevalence
The prevalence of obesity has increased dramatical y during the last decades worldwide. Between 1980 and 2008, the world prevalence has been reported to have doubled from 5% to 10% among adult men and from 8% to 14% among women.4 The largest rise was seen in Oceania in both sexes, while the trend was almost flat for women in central Africa, central and eastern Europe and for men in southeast Asia during this time.4 In 2008, it was estimated that 1.5 bil ion adults had a BMI ≥25 and 500 mil ion of these were obese worldwide, with the highest prevalence of obesity in men seen in the United States (US) and in women in southern Africa.4 According to the latest measured data from the US National Health and Nutrition Examination Survey (NHANES) in 2009-2010, almost 70% of the adult population was overweight or obese, of which half (36%) was obese.5 In Sweden during the same time, 45% of the adult population was overweight or obese, of which one quarter (11%) was obese, according to self-reported data from Statistics of Sweden.6 Self-reported data should, however, be interpreted with some caution, due to risk of systematic underestimations.7 In both the US and Sweden, the most rapid increase of obesity during the last decades has occurred in the morbidly obese group (BMI ≥35).8 9 Several reports have suggested that the prevalence of obesity has levelled off since the early 2000s. For example, the prevalence has remained stable among women in the US since 1999, but not among men.10 In an extensive review of the subject, Rokholm et al11 concluded that a stabilisation or levelling off in the prevalence of obesity was seen in the majority of studies among children and adolescents. In adults the results were diverging, with some of the studies reporting stability, while increases were still observed in others. Despite a level ing of or not, the current prevalence is at al time high. 1.1.3 Health Consequences
Overweight and obesity are associated with several adverse health consequences such as type 2 diabetes, cardiovascular disease including hypertension and hyperlipidemia, musculoskeletal disorders, obstructive sleep apnoea, psychiatric il ness and several cancers (such as postmenopausal breast, colon, kidney, oesophagus cancers).1 12 Some of these co-morbidities increase the risk of death, while others decrease quality of life or ability to work,13-15 causing a major economic burden to society. Obesity alone has been estimated to be responsible for 0.7-2.8% of health care expenditures worldwide,16 while in Sweden, the health care costs of obesity was in 2003 estimated to 3.6 billion SEK, that is 1.9% of the national health care expenditure.17 Obesity increases the risk for al -cause mortality,18 19 20 while some controversy exists for this association in overweight.20 21 It has been estimated that a BMI of 30-35 reduces life expectancy by two to four years, and a BMI of 40-45 with eight to ten years.18 In US adults, diet and activity patterns were already in 2000 ranked as the second most important modifiable risk factor for preventable deaths after smoking, accounting for 17% and 18% of excess annual deaths, respectively.22
1.1.4 Treatment
The main treatment options for obesity include lifestyle modification, very low energy diet (VLED),
pharmacotherapy and bariatric surgery (weight loss surgery). These treatments wil be discussed
below, and VLED in particular in section 1.2.
1.1.4.1 Lifestyle Modification
Lifestyle modification is always recommended as the first-line treatment option for overweight and obesity and includes dietary, physical activity and behavioral modification. The foundation of weight loss includes a negative energy balance over time that is achieved by decreasing the energy intake and/or increasing energy expenditure. The individual effect of behavioral treatment, diet, physical activity has been studied widely, but should according to the body of evidence be combined for the best treatment effect. A meta-analysis by Franz et al23 concluded that diet and physical activity alone resulted in a modest weight loss after one year of -5 kg (-5%) and -1 kg (-1%), respectively. However, the weight loss was greater when combined -8 kg (-9%). In addition, the Look AHEAD (Action for Health in Diabetes) study of subjects with diabetes, reported one year weight losses of -9% of initial body weight in the intensive lifestyle intervention that included diet, physical activity, and behaviour modification,24 and -6% of initial weight after four years.25 1.1.4.1.1 Dietary Strategies Energy deficient diets are often aiming at a 500-1,000 kcal deficit of required energy intake per day to produce a weight loss of approximately 0.5-1 kg/week.26 The impact of dietary macronutrient composition on weight loss has been investigated extensively and potential effects of high-protein diets, low-carbohydrate diets, high-fat diets, and low glycemic index, have been studied. One meta-analysis27 and one review28 comparing low-fat with low-carbohydrate diets both found that low-carbohydrate diets achieved greater weight loss during the first 6 months, but this difference was not present after 12 months. The authors of both papers concluded that the initial greater weight loss may have been linked to adherence, since a higher rate of adherence to low-carbohydrate diets was seen during 6 but not at 12 months, compared to the low-fat diet. In addition, large randomised control ed trials with different diet compositions have reported similar effects on weight loss after two years.29-31 Data from these studies suggests that restricting total energy intake and adherence is more important than diet macronutrient composition for achieving weight loss. For weight loss maintenance, however, there are data suggesting that diet composition could be important. The National Weight Control Registry (NWCR) in the US, containing over 4,000 individuals who have maintained a weight loss of 13.6 kg for a minimum of one year, found that a diet low in energy and fat was associated with long-term maintenance, according to self-reported data.32 In addition, a large randomised control ed European study found that a modest increase in protein content and a modest reduction in the glycemic index, after an 800 kcal/day diet, led to improvements in study completion and maintenance of weight loss.33 1.1.4.1.2 Weight Loss Maintenance In obesity treatment the greatest chal enge is weight loss maintenance. Wing and Hil 34 have defined successful weight loss maintenance as an intentional weight loss of >10% that has been maintained for at least one year. Factors that have been associated with long-term weight loss maintenance, according to self-reported data from the NWCR, are:32 35 36 1) engaging in high levels of physical activity 2) eating a diet that is low in energy and fat 3) eating breakfast 4) self-monitoring weight regularly 5) maintaining a consistent eating pattern across the week 6) attending treatment sessions regularly Factors associated with maintenance after weight loss with VLED are described in section 1.2.4.5. 1.1.4.1.3 Commercial Weight Loss Programmes In addition to traditional obesity treatment within the health care system, commercial weight loss programmes are being used annual y by mil ions. Randomised control ed studies evaluating the efficacy and safety of different commercial programmes are scarce.37 The Weight Watchers programme has, however, been evaluated in three randomised controlled studies.38-40 In a recent study41 it was found that participants in the Weight Watchers group lost twice as much in weight after one year, compared to those in the standard care group (-4 vs -2 kg, with baseline carried forward for missing data).
1.1.4.2 Anti-Obesity Drugs
Anti-obesity drugs are (or have been) indicated in subjects with a BMI ≥30 or a BMI ≥27 with medically complicated obesity42 who have failed to lose weight through lifestyle modification alone. Anti-obesity drugs should always be given in conjunction with a lifestyle modification programme.
1.1.4.2.1 Anti-Obesity Drugs on or Recently Withdrawn from the Market
At present only one anti-obesity drug, orlistat, is available after withdrawal of rimonabant and
sibutramine. The characteristics, mechanisms, weight loss and adverse events of the drugs are
presented in Table 3.
Table 3 Characteristics of anti-obesity drugs
Drug
Mechanism
Weight loss43
Adverse Events
Trade Name
cannabinoid CB1 antagonist induces satiety, increases depression and risk of energy expenditure, decreases suicide ideation lipogenesis Sibutramine
dopamine reuptake inhibitor Reductil/Meridia induces satiety, pressure and pulse rate increases energy expenditure Orlistat
lipase inhibitor reduces dietary fat absorption gastrointestinal Information from references.42-45 Weight loss from a meta-analysis of pooled studies at one year. Al participants in the weight loss trials, including those in the placebo-group, received comprehensive lifestyle education together with approximately a 500 kcal deficient diet. Rimonabant was never approved in the US, and was withdrawn in Europe in 2008 after two years on the market due to reports of severe depression and increased risk of suicide ideation.44 46 Sibutramine was withdrawn in Europe and the US in 201044 after 11 and 13 years on the market, respectively. The reason for the withdrawal was that the Sibutramine Cardiovascular Outcomes trial (SCOUT)47 found that subjects with pre-existing cardiovascular conditions treated with sibutramine, that is subjects with contraindications for the drug, had an increased risk of nonfatal heart attacks and nonfatal stroke. Orlistat has been approved since 199842 and was recently also approved, in Europe and US, as an over-the-counter drug under the trade name Al i, but with half the dosage (60x3mg/day).48 In Sweden Xenical is indicated in subjects with a BMI ≥28 with co-morbidity or a BMI ≥30,26 while Al i in al with BMI ≥28. 1.1.4.2.2 Potential New Candidates - Qnexa, a combination of phentermine and topiramate, was recently (February 2012) recommended to be approved as an anti-obesity drug in the US, but the final decision from the Food and Drug Administration (FDA) is awaiting.44 Reported placebo-adjusted weight losses range between -8.9 to -10.9 kg.44 Liraglutide, an injectable glucagon-like peptide-1 (GLP-1) receptor agonist, is approved under the trade name Victoza for treatment of diabetes. Liraglutide also induces weight loss with reported placebo-adjusted weight loss after one-year of -5.8 kg.49 Contrave, a combination of bupropion and naltrexone, with placebo-adjusted weight losses of -4.2 to -5.2 kg.44 Cetilistat, a newer lipase inhibitor (as orlistat) that has been reported to be associated with less gastrointestinal complications than orlistat, but similar weight loss.44
1.1.4.3 Bariatric Surgery
Bariatric surgery is indicated in obese subjects who have been unable to lose weight through lifestyle
change alone, or in combination with anti-obesity drugs. The BMI indication in most guidelines is a BMI
>40 kg/m2 or a BMI >35 kg/m2 with obesity-related co-morbidity.50 In Sweden the current national
guidelines stipulate a BMI >35 kg/m2 with no further requirements regarding co-morbidity.51
Broadly, three classes of surgery exist: restrictive (e.g. gastric banding), malabsorptive (e.g. biliopancreatic
diversion), and combined procedures (gastric bypass). Table 4 outlines the principles, approximate
weight loss and adverse events of the two most common procedures, global y.50
Table 4 Characteristics of gastric banding and gastric bypass
Procedure
Class
Principle
Weight loss52 Main Adverse Events
Gastric Banding Adjustable band placed around the upper part
Band erosion or migration Restrictive of the stomach, creating a smal pouch Frequent vomiting Limiting the amount of food consumed Gastric pouch dilatation Reservoir leak The stomach is divided by staples creating a small Dumping syndrome Gastric Bypass
pouch to which the smal intestine is attached, Intestinal obstruction Restrictive and while the first part of the intestine is bypassed Nutritional deficiency malabsorptive In addition to the restrictive and malabsorptive Staple line leak mechanism, increased levels of the gut hormones GLP- 1 and PYY are seen which increase satiety Information from references.50 52 53 GLP-1=Glucagon-like peptide-1; PYY= Peptide YY. Gastric bypass leads to greater weight loss and weight loss maintenance compared with gastric banding.52 54 In the Swedish Obese Subjects (SOS) study, the largest weight loss was seen the first and second year, and was fol owed by a weight regain that leveled off between year eight and ten.52 In addition to weight loss, several other beneficial long-term effects are seen after bariatric surgery, such as reduced mortality52, reduced incidence of diabetes and cardiovascular events, major improvements or recovery in preexisting diabetes, hyperlipidemia, hypertension, obstructive sleep apnoea,54-56 improved health-related quality of life,57 and a reduced incidence of cancer.58 Until 2002 about 700 operations were performed annual y in Sweden. Since then, an explosive growth has occurred, with 8,000 surgeries in 2010, of which 98% were gastric bypass procedures.59
1.2 VERY LOW ENERGY DIETS
Very low energy diets (VLEDs) or very low calorie diets (VLCDs) are defined as diets containing 450-800 kcal/day (1.9-3.3MJ/d) or <800 kcal/day (<3.3MJ/d) according to European60 and American reports, respectively.61 62 The diets are designed to be used as the sole source of nutrition and contain the recommended daily al owance for vitamins, minerals, electrolytes and essential fatty acids. Low energy diets (LEDs) or low calorie diets (LCDs), contain between 800 and 1,600 kcal/day (3.3-6.7MJ/day) depending on definition,60 63-65 and often some of the regular meals during the day are substituted by liquid meal replacements. 1.2.1 History
The commercial liquid VLED formula was introduced in the 1970s. Unfortunately, the first products contained low quality protein and were deficient in vitamins and minerals, leading to several fatal dysrhythmias.61 Modern VLEDs, containing high quality proteins and essential nutrients, were introduced in the 1980s and since then no deaths related to VLED have been reported in Europe, according to the European SCOOP-report on VLED use.60 In addition, extensive studies on cardiac function have not found any adverse cardiac reactions after VLEDs.61 66 1.2.2 Composition
VLEDs are not defined as pharmaceutical agents, but as "foods for special medical purposes" with
standardised values regarding their nutritional composition.60 67 The standardised composition
according to the SCOOP-report on VLED use is shown in Table 5.
Table 5 Composition of very low energy diets (VLEDs)60
Nutrients
Content/day
≥50 grams of high quality ≥7 grams , including essential fatty acids (linoleic- and alpha-linolenic acid of resp. 3.0 and 0.5 g) Vitamins and minerals recommended daily intake
VLEDs are generally strict liquid diets provided in powder form and mixed with water before consumption. Depending on the product, 3 to 6 sachets per day are consumed, replacing all meals. A more liberal VLED al ows a restricted intake of other foods such as vegetables. VLEDs could also be based on normal food by combining lean meat, fish and fowl with vitamin and mineral supplements.66 Studies have, however, found that strict adherence to a liquid VLED results in greater weight loss, both as compared to a more liberal approach al owing other foods68 and as compared with normal food.69
1.2.3 Availability
1.2.3.1 Europe
In Europe VLEDs are available over-the-counter, except in Germany and France. In Germany VLEDs are used only under medical supervision, while in France VLEDs are only available on prescription. In other European countries VLEDs are freely available.60 To ensure a safe use of VLEDs, the manufacturers should, according to the SCOOP-report,60 include the fol owing information for the consumer: 1) VLEDs should not be used for longer than three weeks without medical supervision 2) water intake should be at least 1.5 to 2 litre per day (in addition to that mixed with the powder) 3) a warning that VLEDs are unsuitable for children, adolescents, pregnant or lactating women and that elderly and patients with co-morbidities should consult their physician before starting a VLED
1.2.3.2 United States
In the US, recommendations state that VLEDs should only be used under strict medical surveil ance, managed by a physician. VLEDs should also be a part of a comprehensive intervention including medical monitoring and a lifestyle modification programme, preferably together with a dietician, psychologist and/or exercise physiologist.61 65 1.2.4 Treatment
1.2.4.1 Indications
According to the American report National Task Force on the Prevention and Treatment of Obesity,61
VLED may be indicated in wel -motivated individuals with a BMI >30 who have failed in previous weight
loss attempts, or in individuals with a BMI ≥27 with obesity-related co-morbidity.
The European SCOOP-report does,60 however, not report a unified indication. The report only states
that VLED should be used in the treatment of obesity. In hospital-based obesity treatment programmes
in Sweden, the reported indication is a BMI ≥30 or a BMI >27 with co-morbidity.70 In addition, VLEDs
could be indicated in situations where a rapid weight loss is clinical y important, for example before
bariatric surgery or other surgery, to reduce surgical risk and complications.66 71
1.2.4.2 Contraindications
There is no total agreement about VLED indications and contraindications. Table 6 lists the most
common contraindications presented in European60 and American reports.61
Table 6 Common contraindications to very low energy diets (VLEDs)60 61
Contraindications
Relative Contraindications
Behavioural disorders (bulimia, anorexia, alcoholism or drug addiction) Children and adolescents For children who are stil growing VLED should be avoided. However, in selected cases of severe obese children, where other treatments have failed, VLED could be used under strict medical supervision.60 61 Cardiovascular or cerebrovascular disease Gal bladder disease Liver disease and kidney disease There are few reports on the effect of VLED in people over 65. Since Major psychiatric disorder elderly persons already are at increased risk for negative nitrogen due to Major depression normally depleted lean body mass, the increased risks must be balanced Type 1 diabetes mellitus with the benefits of weight loss in older persons.61 66 Pregnant and lactating women The SCOOP-report60 also lists: Acute ischaemic cardiopathies, electrolyte disorders, gout, haemopathy, hereditary metabolic diseases, major surgery or serious accident within the last 3 months, orthostatic hypotension, porphyria. The American National Task Force on the Prevention and Treatment of Obesity61 also lists: Systemic infection or disease causing protein wasting.
1.2.4.3 Programme
The duration of a VLED-programme in a hospital-based obesity treatment programme usually range
between eight and 16 weeks,66 and performed in conjunction with lifestyle modification. After the
VLED-period, a two to six week re-feeding period often fol ows to gradually introduce normal food
again. The reasons for this are to prevent abrupt retention of fluid, to prevent abdominal discomfort
and to adopt strategies to adjust eating behaviour. A longer re-feeding period has, in a randomised
control ed trial,72 been associated with better weight loss maintenance. The re-feeding period should
be fol owed by a weight loss maintenance programme to prevent relapses in weight (further details in
section 1.2.4.5).
1.2.4.4 Weight Loss
The amount of weight loss differs between subjects since the energy content of VLEDs is fixed and does not take sex, age, weight or lean body mass into account, which al are determinants of basal metabolic rate. Weight loss depends on the specific person's energy expenditure. For example, a 700 kcal/day VLED will lead to a much smal er energy deficit (and weight loss) in a sedentary female compared to an active male. Additional y, severely obese persons who expend more energy will lose more weight compared to moderately obese persons. An alternative definition of VLED has been suggested as approximately 10 kcal/kg of desirable body weight,73 or <50% of an individual's predicted resting energy expenditure.65 However, these definitions have not been used in practice. Due to the above mentioned factors weight loss could differ substantially between individuals. In general VLED results in an average loss of 1.5-2.0 kg/week in women and 2.0-2.5 kg/week in men.61
1.2.4.5 Weight Loss Maintenance after VLED
For all obesity treatment programmes weight loss maintenance is the greatest chal enge. For VLED in particular, the effect of long-term weight loss maintenance has been questioned due to the reported poor long-term maintenance. In a meta-analysis65 of six head-to-head studies comparing the long-term effect of VLEDs compared to LEDs, Tsai and Wadden concluded that VLEDs resulted in significantly greater short-term weight losses (-16% vs -10% over 13 weeks; p <0.001), but the maintenance at two years was poor (-6% vs -5%; p >0.2). However, neither of these trials included an extensive exercise programme,74 75 pharmacotherapy,76-81 a prolonged re-feeding period72 75 or a low glycemic index and high protein diet33 as a part of the maintenance programme, which are al proven to limit weight regain. In addition, early large weight loss has been shown to be a predictor of long-term weight loss maintenance in a meta-analysis.82 1.2.5 Side-Effects
Common side-effects of VLED include diarrhoea, constipation, headache, nausea, vomiting, dizziness, orthostatic hypertension, dry mouth, poor cold tolerance, dryness of skin and loss of hair.60 61 These side-effects are all transient and related to the semi-starvation during VLED, but also due to inadequate fluid intake.60 More serious side-effects, but not as common, are gallstones and gout.61 65 66 83 Cardiac complications were, as previously described in section 1.2.1, a side effect with the old incomplete liquid formulas but not with the modern VLEDs.
As the risk of gal stones during treatment with VLED compared LED wil be evaluated in Study I I of
this thesis, a further description of gal stones and their association to obesity is provided below.
1.2.5.1 Gallstones
Gal stones (cholelithiasis) develop in the gal bladder, and are clusters of crystal ised pieces of bile consisting either of cholesterol or bilirubin, with cholesterol stones being the most common type in western countries.84 Multiple factors interact causing gallstone formation, the most commonly described mechanisms are:84 1) Super-saturation of bile with cholesterol leading to cholesterol crystal isation Supersaturation occurs if the liver excretes more cholesterol than the bile can dissolve, leading to aggregation of excess cholesterol into crystals that eventual y form into gal stones. 2) Insufficient gal bladder emptying due to impaired motility Reduced ability to empty the gal bladder completely, or often enough, may result in concentrated bile, causing cholesterol aggregation and formation of gal stones. 1.2.5.1.1 Risk Factors, Clinical Spectrum and Treatment Gal stone formation may be influenced by genetic and environmental factors, and common risk factors include: female sex, obesity, higher age (>40y), ethnic origin, and rapid weight loss.84-86 Gal stones may be asymptomatic or symptomatic. Asymptomatic gallstones are also cal ed "silent gallstones", and treatment is not recommended if these gallstones are found.84 Approximately 15-20% of the asymptomatic gal stones develop into symptomatic stones.86 Symptomatic gal stones manifest as attacks of biliary colic, lasting from 30 minutes to 6 hours, and typically occur 15 minutes to 2 hours after food intake. The primary treatment is analgesics drugs, with diclofenac being the most common. Cholecystectomy may be performed if the symptoms recur frequently, if the gal bladder wall has become calcified, or if the gal bladder bile ducts or pancreas have been inflamed.84 1.2.5.1.2 Obesity and Rapid Weight Loss Obesity in itself is a risk factor of developing gallstones and the increased risk is particularly evident in women, but studies in men have also shown an increased risk.87-89 Weight loss also increases the risk for gal stones, especially after rapid weight loss with VLED or bariatric surgery.87 88 The underlying mechanism for the increased risk of gallstones among obese persons may be associated with increased levels of cholesterol in the bile, that eventual y leads to super-saturation of the bile.86 87 88 Rapid weight loss may increase the risk of gallstones by the same mechanism, that is by increased levels of cholesterol, but also by impaired gallbladder motility. The impaired motility during VLED is thought to be caused by reduced gal bladder stimulation due to the low fat content of a VLED.87 89 A majority of the clinical studies that have found an increased risk of gallstone formation during VLED have been American studies conducted in the late 1980s and early 1990s, using VLEDs with low levels of fat (≈1 g/day).90-94 In formulations containing higher amounts of fat (12-30 g/day), the incidence of gallstones has been much lower.95-98 In a review, Festi et al89 concluded that adequate fat content is important in gallstone prevention, with 10 g of fat/day as a threshold for obtaining efficient gal bladder emptying. A lower recommendation of 7 g fat/day has, however, been given by the European SCOOP-report on VLED use.60
1.3 OBESITY AND OBSTRUCTIVE SLEEP APNOEA
Obstructive sleep apnoea (OSA) is one of the most common sleep disturbances, affecting an estimated 24% of middle aged men and 9% of women, according to data from the Wisconsin Sleep Study.99 In the same study, OSA with clinical symptoms as excessive daytime somnolence, that is obstructive sleep apnoea syndrome (OSAS) occurred in an estimated 4% and 2% of middle aged men and women, respectively.99 1.3.1 Definition, Risk Factors, Symptoms and Treatment
1.3.1.1 Definition
OSA is caused by obstruction of the upper airway, either by total blockage (apnoea) or partial blockage
(hypopnoea) of airflow for at least 10 seconds during sleep.100 101 The apnoea-hypopnoea index (AHI),
which measures the average numbers of apnoeas and hypopnoeas per hour of sleep, is used to classify
the severity of OSA, according to the American Academy of Sleep Medicine (Table 7).100 Occurrence
of OSA is defined as an AHI ≥5 events/h.100 The diagnosis is confirmed by a sleep study, either by an
overnight laboratory polysomnography, or a by an ambulatory polygraphy equipment at home.
Table 7 Classification of obstructive sleep
apnoea (OSA)100
Severity
AHI (events/hour)
Moderate
AHI=Apnoea-hypopnoea index.
1.3.1.2 Risk Factors
A variety of factors, genetic as well as environmental, increase the risk of OSA. Frequently described
risk factors include: male sex, increased age, obesity (especially abdominal), ethnicity, heritability,
smoking, and alcohol consumption.101-103 Body weight has widely been described as the strongest risk
factor for OSA,101 104 and data from the three largest epidemiological sleep studies, that is The Sleep
Heart Health Study,105 The Wisconsin Sleep Cohort Study,106 and The Cleveland Family Study107, have
al found weight gain to be associated with increased risk for OSA. Additionally, reports show that the
majority (60-70%) of persons with OSA are either overweight or obese.101 103 108
1.3.1.3 Symptoms and Consequences
Pauses in breathing caused by upper airway obstruction leads to hypoxemia, arousals and increased sympathetic activity, causing sleep fragmentation and potential y leading to adverse health outcomes. Associated features during sleep include loud snoring, frequent arousals, gasping and nocturia. Daytime symptoms due to sleep fragmentation are sleepiness, fatigue, impaired cognitive function, morning headache and impotence.109 Left untreated, OSA has been described to be associated with an increased risk of hypertension, cardiovascular and cerebrovascular diseases, decreased insulin resistance, metabolic syndrome, al -cause mortality, reduced quality of life and working capacity. Due to daytime sleepiness OSA also increases the risk for driving- and occupational-related accidents.101 110-112 103
1.3.1.4 Treatment
The most commonly used treatment for OSA is continuous positive airways pressure (CPAP), a nasal mask connected to a compressor that keeps the airways open by a mild air pressure. The CPAP effectively relieves symptoms but has no or very short curative effect.109 This also holds for treatment with oral devices mostly used for mild to moderate OSA. An oral appliance protrudes the lower jaw and thereby widens the airway. Uvulopalatopharyngoplasty is a surgical procedure where the tonsils and parts of the soft palate are removed. The method can be curative for the patient with correspondent anatomical conditions.109 Lifestyle modification including weight loss should, according to guidelines, always be recommended in overweight or obese patients.113 The beneficial effect of weight loss on OSA was recently demonstrated in three randomised control ed trials, one of which is included in this thesis (Study I), thus providing the first high quality evidence of the effectiveness of weight loss.114-116 1.3.2 Mechanisms
The epidemiological association between obesity and OSA is well documented,99 100 but the mechanisms remain unclear.101 102 Potential mechanisms include anatomical alterations caused by a mechanical load effect and/or disturbance in upper neuromuscular control caused by humoral factors: 1) Mechanical Load Effect: Due to fat accumulation in specific sites surrounding the upper airway, the thorax and abdomen, obesity may lead to OSA by altering airway anatomy and respiratory control. These alterations could lead to narrowing of the upper airway structure, reduced chest wall compliance, reduced lung volume, reductions in functional residual capacity and reduced tracheal traction, leading to upper airway narrowing, col apse and airflow obstruction.104 108 2) Adipokine Effect: It has been suggested that factors other than pure mechanical load may contribute to the pathogenesis of OSA.104 Humoral factors, adipokines, produced by the metabolic active visceral adiposity may influence upper airway function. Most widely discussed is leptin, which in addition to regulation of food intake also regulates respiratory control. Also discussed are proinflammatory cytokines that may lead to alterations in upper airway function, causing disturbance in neuromuscular control, and increasing the risk of OSA.104 113 117 1.3.3 Previous Research regarding Weight Loss and Treatment
Weight loss has long been recommended as treatment option for overweight and obese patients with
OSA, but without high quality evidence, and unclear compliance (both from treating doctors, and
patients receiving weight loss advice).118 119 The association between weight and AHI change has been
wel described in large epidemiological sleep cohort studies.105-107 However, it was first in 2009 that
evidence from randomised control ed trials supporting this concept was published (Table 10).114-116
Previously published clinical studies had several methodological limitations, including lack of either
randomisation,120-122 or control group for comparisons123-137 and limited fol ow-up (Table 8-10).119
1.3.3.1 Uncontrolled Studies
Table 8 and Table 9 presents uncontrol ed studies of weight loss by surgery or a dietary intervention
published between 1985 and 2010 (Study I in this thesis is included). The number of included subjects,
duration and weight loss methods have differed between the studies; however all have found weight
loss to be associated with improvements in OSA. Dietary weight loss studies have found a 24-68%
reduction of AHI or ODI4 with a weight loss (kg) between 8-14% of initial body weight, and surgery
studies AHI improvements of between 49-78% with a weight loss of 29-37%.
Table 8 Uncontrolled studies of dietary weight loss and obstructive sleep apnoea (OSA)
Article
Weight loss* AHI/ODI
reduction
Nerfeldt138
LED; 800 kcal/day
96weeks lifestyle) VLED; 554 kcal/day
43weeks lifestyle) Barnes123
VLED; n/a
Australia 8weeks re-feeding) LED; 1,000-1,500
kcal/day
12weeks lifestyle) Kajaste129
VLED; 500 kcal/day
(6weeks VLED+90weeks Hakala127
VLED; 500 kcal/day
Lojander133
VLED; 500 kcal/day
46weeks lifestyle) Kansanen131
VLED; 600-800
kcal/day
Kajaste130
Noseda134
Dietary Advice (N=36) or
Bariatric Surgery if
Suratt136
VLED; 420-800
kcal/day
* BMI reduction is given if weight loss in kg is not available. AHI=apnoea-hypopnoea index; CBT=cognitive behavioural treatment; Comp=completers analysis; ITT=intention-to-treat (missing data imputed); Lifestyle=behavioural modification programme; n/a=not available; ODI4=oxygen desaturation >4%/h of sleep.
Table 9 Uncontrolled studies of bariatric surgery and obstructive sleep apnoea (OSA)
Article
Follow-up
Weight loss,*
(months)
reduction
Lettieri132
2008
Haines126
2007
Fritscher125
2007
Dixon124
2005
Australia Valencia-Flores137
2004
Pillar135
1994
Israel * BMI reduction is given if weight loss in kg is not available. AHI=apnoea-hypopnoea index; GBP=gastric bypass; n/a=not available; RDI=respiratory disturbance index.
1.3.3.2 Controlled Studies
Although control ed studies were published before 2009, none of these trials were randomised.120-122
During 2009 three randomised studies were published. Tuomilehto et al115 investigated the effect of
VLED fol owed by supervised lifestyle counselling for one year in overweight or obese patients with
mild obstructive sleep apnoea (Table 10). Thereafter Foster et al116 investigated the effects of
intensive lifestyle change in overweight or obese patients with type 2 diabetes and mild to severe OSA
for one-year. Finally, we investigated the effects of VLED during nine weeks in obese men with
moderate to severe OSA (Study I).114
Al three trials found weight loss to result in clinical y relevant AHI improvements. In 2010,
Tuomilehto et al published a two-year fol ow-up study,140 demonstrating that although there was some
weight regain OSA remained improved and did not fol ow the trend of modest weight gain.
Table 10 Controlled studies of weight loss and obstructive sleep apnoea (OSA)
Article
start (months)
Randomised controlled trials
Tuomilehto115 1.VLED;600-800 kcal/day
(12weeks+lifestyle 40weeks) 2. Lifestyle only
Tuomilehto140 1.VLED;600-800 kcal/day
(12weeks+lifestyle 92weeks) 2. Lifestyle only
1. Intensive lifestyle
2. Diabetes education
2. Controls
Controlled trials (non-randomised)
1. Calorie restriction
2. Matched controls
Australia 1. Calorie restriction
2. Matched controls
+0.1kg/m2 (+0.3%) 1. Bariatric surgery
2. Matched controls
AHI=apnoea-hypopnoea index. *AHI was not measured, but a sleep questionnaire was used to evaluate the persistence of apnoeas. 2 OBJECTIVES
2.1 OVERALL OBJECTIVE
The overal objective of this thesis was to evaluate effects and side-effects of VLEDs in different obesity treatment settings, and to characterise discontinuation in obesity treatment programmes. 2.2 SPECIFIC OBJECTIVES
1. To evaluate a weight loss programme as treatment option for patients with obstructive sleep Firstly: to evaluate the effect of weight loss induced by a VLED on OSA in a randomised control ed trial (Study I) Secondly: to determine whether initial improvements in OSA after the VLED were maintained after one year in a observational study (Study I ) 2. To assess the risk of gallstones requiring hospital care, and cholecystectomy, in a commercial weight loss programme using VLED or LED (Study I I) 3. To characterise overal discontinuation and discontinuation due to adverse events in obesity treatment programmes by analysing data from anti-obesity drug trials (Study IV)
2.3 RATIONALE FOR INCLUDED STUDIES
2.3.1 Study I&II: Weight Loss and Obstructive Sleep Apnoea
Few treatment options are available for OSA. The most commonly used strategy to facilitate breathing during sleep and to reduce morbidity and mortality is CPAP.109 Although weight loss has long been advocated as a primary treatment strategy for the condition,113 at the time of the study initiation/planning (spring 2008) no randomised control ed trials existed to support this concept.118 119 The rationale for Study I was, therefore, to assess potential improvement in OSA after VLED-induced weight loss in a randomised control ed trial including obese male patients with moderate to severe OSA, a patient group with increased mortality risk.111 112 The rationale for Study II was to evaluate the extent to which initial improvements in OSA after the VLED-induced weight loss were maintained after one year. 2.3.2 Study III: Risk of Gallstones during VLED
Each year millions use commercial weight loss programmes, including intensive treatment schemes such as VLEDs.141 Safety concerns exist for VLEDs, especially regarding gallstone development.88 The magnitude of the risk is unclear: clinical studies evaluating the risk of gal stones associated with VLED have primarily been conducted in the late 1980s and early 1990s and using VLEDs containing low levels of fat (≈1 g/day).90-94 The few existing studies with VLEDs containing higher amounts of fat60 show a much lower incidence of gal stones.95-98 Major limitations of all published studies are lack of control groups, smal sample sizes and short fol ow-up. The rationale for this one-year cohort study conducted in a real-world setting was to investigate the risk of gallstones requiring hospital care, and cholecystectomy, during treatment with VLED (fat content 7-9 g/day) compared to matched controls using LED in a multi-centre commercial weight loss programme. 2.3.3 Study IV: Treatment Discontinuation
Weight loss treatment programmes in observational settings (hospital-based and commercial) as wel as randomised trials are afflicted by high levels of attrition. In a systematic review of weight loss studies addressing factors associated with attrition,142 one-year dropout rates of diet and lifestyle modification studies ranged between 16% and 77%. Pharmacotherapy trials could be used as benchmark of discontinuations rates in obesity treatment programmes, since these are strictly controlled and generally have significant resources to minimise attrition. The rationale for Study IV was to characterise overall discontinuation, but also discontinuation due to adverse events and lack of effectiveness, in obesity treatment programmes by pooling data from randomised control ed anti-obesity drug trials. 3 METHODS
3.1 STUDY DESIGNS AND STUDY POPULATIONS
Four different study designs and three different populations were included in this thesis (Table 11).
Table 11 Study design of included studies
Study Design
Population
Randomised controlled trial
Obese Swedish men with moderate to severe OSA Observational follow-up study
undergoing a hospital-based weight loss programme Matched register-based cohort
Overweight and obese adult Swedish men and women enrol ed in a commercial weight loss programme Pooled data from published randomised control ed trials, enrol ing overweight and obese men and women, eligible for anti-obesity drugs OSA=Obstructive sleep apnoea 3.2 ETHICAL CONSIDERATIONS
In the clinical study of the effect of weight loss on OSA (Study I&I ) obese patients were recruited from a patient database at the Aleris FysiologLab sleep clinic in Stockholm, Sweden. The patients who declared an interest in participating and met the inclusion criteria gave written informed consent to participate in the study. Al patients were given a study identification number which was used throughout the study. The identification lists were kept in a locked room, with no access for research staff. Ethical approval was granted by the regional ethical review board, Stockholm, Sweden (reference number: 2008/1634-31). In the commercial weight loss study (Study III) the database used by the commercial vendor to track customer progress and compliance was used to retrieve participant data. By using the unique personal identification number assigned to each Swedish resident, these data were linked on an individual level to the National Patient Register, the Causes of Death Register and the Prescribed Drug Register. The integrity of the participants should always be considered when doing register-based research. For integrity protection purposes, the National Board of Health and Welfare anonymised all data before returning them to the research group. Furthermore, data were only presented on an aggregated level. Ethical approval was granted by the regional ethical review board, Stockholm, Sweden (reference number: 2010/1059-31/1). In the meta-analysis (Study IV) only previously published studies were analysed of which each individual study already reported that ethical permission was granted, hence ethical approval was not applied for.
3.3 STUDY I&II: WEIGHT LOSS AND OBSTRUCTIVE SLEEP APNOEA
The study was of one-year duration and consisted of a weight loss phase (0-9 weeks, RCT) and a weight loss maintenance phase (>9-52 weeks, observational) carried out between February 2009 and April 2010 at a specialist outpatient obesity clinic (hospital-based). 3.3.1 Participants and Design
63 men aged 30-65 years with BMI 30-40 and moderate to severe obstructive sleep apnoea (OSA)
defined as an AHI ≥15 events/hour, al treated with CPAP, were included in the study. Subjects were
randomly assigned to intervention (weight loss by a VLED programme) or control groups in a 1:1 ratio.
To reduce the likelihood of controls dropping out, control subjects were also offered the same
treatment once the nine-week fol ow-up as controls was completed. After the weight loss programme,
al patients were offered a standard care hospital-based outpatient weight loss maintenance
programme (Figure 1).
Figure 1 Design and
treatment periods in obese men with moderate to severe obstructive sleep apnoea (OSA) Adapted from Johansson, K et al (2011). "Longer term effects of very low energy diet on obstructive sleep apnoea in cohort derived from randomised control ed trial: prospective observational fol ow-up study." BMJ 342: d3017. 3.3.2 Intervention
3.3.2.1 The Weight Loss Phase (Nine weeks)
The weight loss intervention consisted of a seven-week VLED, fol owed by a two-week re-feeding
period (Figure 2), while the control group was instructed to adhere to their usual diet.
Figure 2 Energy intake
(kcal/day) during the nine-
week weight loss phase The VLED was a 554 kcal/day (2.3 MJ/day; 4 sachets per day à 138 kcal, 9-11g fat/day) liquid energy intake formula (Cambridge Weight Plan, Northants, UK). To confirm dietary compliance, urinary ketosis was assessed at each visit. During the two weeks of re-feeding a gradual introduction of normal food in a strict manner to reach 1500 kcal/day (6.3 MJ/day) at week nine was carried out. Every other
week a one-hour group session, supervised by a research nurse and the study dietitians, was provided
to build group support and provide motivation.
3.3.2.2 The Weight Loss Maintenance Phase (43 weeks)
The maintenance programme started immediately after the weight loss phase and was based on standard behaviour modification group therapy with a self-help manual.143-146 Behaviour Modification: The programme consisted of monthly three-hour group therapy meetings (10 visits in total). Each group comprised 13-15 patients and was led by a research nurse and a dietitian. The behaviour modification focused on nutrition education, eating behaviour, hunger and craving, relapse situations, and increased physical activity. Other important aspects were evaluation of progress and identification of personal and environmental influences affecting eating and physical activity. In conjunction with the group sessions each patient was seen by a nurse for anthropometry measurements and by a dietitian for individual dietary advice. Energy Intake: During the first two weeks of the maintenance phase the same diet as for the last days of the re-feeding period was fol owed, that is a 1500 kcal/day diet including normal food and one liquid meal replacement. Thereafter each patient's individual energy requirement for weight loss maintenance was calculated according to the Harris Benedict formula.147 The recommended percentage of total energy intake (E%) for fat, carbohydrates and protein fol owed the 2005 Swedish Nutrition Recommendation,148 that is to reduce fat to no more than 30E%, carbohydrates to 55E% (with a maximum of 10E% from pure sugar) and protein to 15E%. To achieve these recommendation the patients were recommended to increase the intake of fruits, vegetables, poultry, fish and lean meat, and by limiting dairy fats, fatty meat, sweets, pastries and desserts. Prevention of Weight Gain: If a patient's weight had increased by more than 2 kg since the last visit, action to prevent further weight regain was taken. The first action was use of partial meal replacement, which included exchange of one or two daily meals with a 138 kcal VLED sachet (Cambridge Weight Plan, Northants, UK). As a secondary option sibutramine or orlistat was prescribed.
3.3.3 Outcomes
3.3.3.1 Obstructive Sleep Apnoea
Sleep measurements were derived from two consecutive nocturnal sleep studies in the home, using a
six channel ambulatory polygraphy equipment (Watch PAT100, Itamar Medical Ltd, Caesarea, Israel).
Patients were carefully instructed not to use their CPAP the two nights before and during the
nocturnal sleep studies. The sleep studies were performed at baseline, after the VLED, and at one year.
The primary outcome was AHI, which is the major disease severity index for OSA. In addition, oxygen
desaturation episodes of 4% or more per hour of sleep, the nadir of arterial oxygen saturation, and
percentage of supine time were recorded. Daytime sleepiness was assessed with the Epworth
sleepiness scale, an eight item self administered questionnaire.
3.3.3.2 Body Composition
Fat loss was assessed with anthropometry and body composition changes from baseline at weeks 1, 3,
5, 7, 9, during the weight loss phase and monthly during the weight loss maintenance phase. Percentage
body fat and body weight were measured with the Tanita BC-418MA body fat analyser. Standing height
was measured to the nearest centimetre with a wal mounted stadiometer. Waist circumference was
measured in duplicate halfway between the iliac crest and the lower rib cage. Neck circumference was
measured in duplicate at the level of the superior border of the cricothyroid membrane.
3.3.3.3 Metabolic Measures
Metabolic variables were measured at baseline, after the VLED, and after one year according to
standard laboratory procedures after 12 hours fasting, including insulin, HbA1c, total cholesterol, LDL,
HDL, triglycerides, TSH, urate, ALAT, creatinine, and glucose. Systolic and diastolic blood pressure
were measured in duplicate after 5-minutes rest in supine position.
3.3.3.4 Quality of Life
Health-related quality of life was measured with the SF-12 health survey to allow quantification of a physical and a mental component score. Scores were compared with quality of life reference data for the male Swedish population.149 3.3.4 Side-Effects
At each visit throughout the study side-effects from the VLED during the weight loss phase and the weight loss maintenance phase were noted by a nurse. The study physician then classified these events for potential causality (unlikely/possibly/likely).
3.4 STUDY III: RISK OF GALLSTONES DURING VLED
This matched cohort study on risk of gallstones requiring hospital care and cholecystectomy after VLED or LED was conducted in the commercial weight loss setting in Sweden. Participant data were retrieved from the database used by the commercial company. By using the unique personal identification number assigned to each Swedish resident, these data were linked on an individual level to the National Patient Register, Prescribed Drug Register and to the Causes of Death Register for fol ow-up of vital status. The National Board of Health and Welfare anonymised all data before sending the linked datasets to the research group. The commercial weight loss programme and the registers are described below. 3.4.1 Weight Loss Programme
3.4.1.1 Participants
The study included consecutively enrolled adult customers (age ≥18 years; n=8,361, after matching
6,640) from the commercial weight loss company Itrim in Sweden (www.itrim.se) from January 1,
2006, until May 31, 2009. Data were col ected from 28 centres across Sweden.
3.4.1.2 Description of the Weight Loss Programme
The weight loss programme was of one-year duration and consisted of an initial three-month weight loss phase fol owed by a nine-month weight loss maintenance phase. During the weight loss phase, the participants were able to select, together with their health coach, one of four programmes (VLED, LED, normal food, or exercise). In this study only the VLED and LED programmes were included, since these are the two programmes including liquid formula products. Although all participants were paying customers and were free to choose weight loss method, the company used specific criteria for VLED use, consistent with the SCOOP-report recommendations.60 3.4.1.2.1 Weight Loss Phase (Three Months) During the weight loss phase the included participants attended either the VLED or the LED weight loss programme: - VLED: Liquid-based formula diet of 500 kcal/day (2.1 MJ/day; 4 sachets per day à 125 kcal, 7-9 g fat/day, Itrim Sweden) for 10 weeks followed by two weeks gradual introduction of normal food. Early introduction of normal food occurred when the participant was either satisfied with the achieved weight loss or had reached normal weight (BMI 18.5-24.9 kg/m2).
-
LED: Two regular meals consisting of normal food and two liquid meal replacements (à 125 kcal, Itrim Sweden) providing a caloric content of approximately 1,200-1,500 kcal/day (5.0-6.3 MJ/day) depending on body size and exercise levels. Both groups attended a one-hour group session every other week to build group support and provide motivation. The group session was led by a trained health coach, and included information on nutrition, exercise, eating patterns, goals and expectations. Also, the participants were recommended to attend two to three weekly workout sessions for at least 30 minutes at the Itrim centre. 3.4.1.2.2 Weight Loss Maintenance Phase (Nine Months) After the weight loss phase, both groups entered the same nine-month weight loss maintenance phase with focus on exercise, but also including behavioural changes, dietary advice and self-monitoring. Behavioural Changes: These were facilitated by a structured programme, including one-hour group sessions every other week during the maintenance phase (20 in total). Each session was supervised by a company trained health coach, who provided encouragement to participants throughout the programme. Each group session covered a specific topic, such as health benefits of weight loss, healthy eating strategies, finding realistic eating and exercise routines, health benefits of exercise, stress management, social support, etc. To further aid behaviour change, there were also four 30 minutes face-to-face counselling sessions with a company trained health coach at baseline, three, six and 12 months. Regular Exercise: All participants were encouraged to continue to work out two to three times per week. Moreover, all participants used a validated pedometer (Yamax SW-200) and were given a tailored plan for increased walking during everyday living, such as walking to and from work. Dietary Advice: These emphasised regular meal patterns, a diet rich in fruit and vegetables, and reducing the amount of dietary fat and sugar. Self-Monitoring: This was facilitated by weight, diet and exercise diaries, including diet and exercise plans, and graphs for plotting weight, waist circumference, planned and completed circuit training sessions, and pedometer-assessed steps/day. 3.4.1.2.3 Programme Cost The cost for attending the one-year programme was approximately SEK 9,000 (≈$1,300/€1,000), excluding liquid formulae diets and meal replacements, and was paid by the participants. Although Itrim headquarters provided all centres with recommendations for programme pricing, each centre was al owed to decide its own programme price tailored to local customer demand, rents for facilities, etc. 3.4.2 National Health Register Data
3.4.2.1 The National Patient Register
The Swedish National Patient Register contains nationwide data on inpatient and non-primary outpatient care visits, including day surgery. The register was started in 1964 when the National Board of Health and Welfare started to col ect information regarding inpatients at public hospitals in selected county councils. Nationwide coverage on all inpatient care was attained in 1987, day-surgery from 1997, and non-primary outpatient care was included in 2001. Primary care data are, however, so far not included. The register includes, among other things, personal identification number, visit date, and main as well as contributory diagnoses coded using International Classification of Diseases (ICD7-ICD10) and procedure codes for surgical interventions.
3.4.2.2 The Causes of Death Register
The Swedish Causes of Death Register contains deaths and causes of deaths of Swedish residents
from 1961. The register contains personal identification number for the deceased, date of birth, date of
death, and sex. The cause of death, with one underlying cause of death and several contributory
causes, is available for >99% of al deaths occurring (including deaths occurring abroad).
3.4.2.3 The Prescribed Drug Register
The Prescribed Drug Register contains information on drugs dispensed on prescription or equivalent in Swedish pharmacies since July 2005. The register contains, among other things, data on the dispensed product (identity, quantity, price) and date of dispensing. ATC codes are used for identification of drugs. 3.4.3 Outcomes
3.4.3.1 Gallstone Problems
The primary outcome was gallstone problems requiring hospital care during the one-year weight loss
programme, while cholecystectomy was investigated as secondary outcome. The primary and
secondary outcomes were retrieved from the National Patient Register (Table 12).
Table 12 Outcomes retrieved data from the National Patient Register
Register Data
ICD 10 or Procedure Codes
Gallstones/Cholelithiasis K80
Cholecystectomy
3.4.3.2 Body Composition
Body composition data were col ected at baseline, 3, 6 and 12 months. Body weight and body fat percentage were measured in a non-fasting state with the Tanita TBF-300 bioelectrical impedance monitor. Waist circumference was measured midway between the iliac crest and the lower rib cage. Height was measured by a wal -mounted stadiometer without shoes. 3.5 STUDY IV: TREATMENT DISCONTINUATION
This study was a systematic review and meta-analysis including published placebo-controlled
randomised trialsa of orlistat (Xenical®), sibutramine (Reductil®) and rimonabant (Acomplia®).
3.5.1.1 Identification and Inclusion of Studies
A systematic search of three bibliographic databases (Medline, EMBASE and Cochrane controlled trials
register) from 1990 to May 7, 2008, was performed to identify articles. The search was limited to
humans, randomised placebo control ed trials, English-language publications and adults. The reference
lists of identified articles were also searched for additional studies, as were reference lists of previously
published systematic reviews. Two authors separately screened the abstracts for inclusion or exclusion
of studies. Ful -text articles were retrieved from al abstracts that were potentially relevant and were
reviewed independently by the two authors. In case of conflicting views, a third person was asked to
resolve matters. The criteria for included studies are presented in Table 13.
Table 13 Inclusion and exclusion criteria of included studies
Study Inclusion
Study Exclusion
Placebo-Controlled RCTs
Weight Loss Maintenance Studies
Licensed Doses for Clinical Use:
Not Licensed Doses for Clinical Use:
- orlistat (3x120 mg/day) - orlistat (180 mg/day) - sibutramine (10–15 mg/day) - sibutramine (>15 mg/day) - rimonabant (20 mg/day) - rimonabant (5 mg/day) Duration 12-24 months
The systematic search resulted in inclusion of 28 trials: 16 studies of orlistat (n=7,038), seven of
sibutramine (n=1,475) and five of rimonabant (n=4,944).
3.5.1.2 Data Extraction and Synthesis
From the included studies data on participants, interventions, discontinuation and reason for
discontinuation were extracted independently by two authors. The individual studies were then
combined in a meta-analysis and the overall risk for discontinuation, lack of effectiveness and
discontinuation due to adverse events were estimated compared to placebo.
3.5.1.3 Interventions in Included Trials
Pharmacotherapy trials are in general highly control ed and fol ow strict protocols according to Good Clinical Practice (GCP) guidelines. Hence, the interventions used in the 28 different studies were similar, with the participants in the active treatment group receiving an individual energy deficient diet (approximately 500 kcal/day deficit) in combination with exercise and lifestyle modification, in addition to the active drug or placebo. a Two of the three anti-obesity drugs on the market when this study was performed have been withdrawn due to adverse events. Rimonabant was withdrawn in 2009 and sibutramine in 2010.
3.6 STATISTICAL ANALYSIS
The analyses conducted and reported in this thesis were performed by using three different statistical
software programmes. The different tests and methods used in the studies are summarised below
(Table14).
Table 14 Statistical tests and programme used in the current thesis
Study I Study II Study III Study IV
Statistical Tests/Methods
Student's T-Test Linear Regression Logistic Regression Conditional Cox Regression Statistical Programmes
SPSS (version17.0, Chicago, IL) Stata (version 10, Col egeStation, TX) SAS Statistical Software (version 9.3, SAS Cary, NC, USA) 3.6.1 Student's T-Test – Continuous Data
Student's t-test is used for normal y distributed data to determine if a difference between two means is greater than that expected by chance. Two-sample t-tests to estimate mean differences can be either unpaired (also cal ed independent) or paired (also cal ed dependent): - Unpaired/Independent: The means of two independent groups are compared Paired/Dependent: The means at two different time points are compared within the same group 3.6.2 – Categorical Data
The chi-square test is used to compare if there is a difference, other than that expected from chance, across categorical variables. The test is only regarded as valid for samples with at least 80% of expected frequencies greater than five. For smaller samples, where chi-squared tests may be invalid, the Fisher's exact test is recommended.
3.6.3 Linear Regression
Linear regression is used to estimate the relationship between a continuous outcome (dependent
variable) and one or more predictor/-s (independent variable/-s). The predictor/-s can either be
continuous, binary, or categorical variables. The regression line is given by the best fitted line through
the observed data:
Y = β + β
where Y is the outcome, β is the intercept, β is the regression coefficient for the X variable/-s,
and X is the predictor variable/-s. 3.6.4 Logistic Regression
Logistic regression is a form of regression which is used when the outcome is a dichotomous variable
(0/1). The independent variables, as for linear regression, can be either continuous, binary, or
categorical. To al ow a linear relationship to be model ed, the outcome variable is transformed into a
logit variable (the natural log of the odds of the dependent variable occurring, or not occurring). In this
way, logistic regression estimates the odds of a certain event occurring:
loge [P/(1 – P)] = β + β
where P is the proportion with the outcome, β is the intercept, β is the regression coefficient for the
X variable/-s (which when back-transformed from the log scale to the natural scale are odds ratios), X is the predictor variable/-s, and loge [P/(1 – P)] is the logit transformation. 3.6.5 Analysis of Covariance
Analysis of covariance (ANCOVA) is a combination of analysis of variance (ANOVA) and linear regression.150 As for linear regression, the outcome variable has to be continuous, and at least two predictors has to be included; one continuous and one categorical. ANCOVA tests whether certain factors have an effect on the outcome variable after removing the variance of the added covariates. 3.6.6 Cox Regression
Cox regression, which uses the proportional hazards model, is designed for analysis of time to event data. Time to event data are generated when the measurement of interest is the time from a wel -defined origin of measurement (for example treatment start) to occurrence of an event of interest (for example cholecystectomy). Time to event analysis is also known as lifetime analysis, and survival analysis. One or more predictor variables are used to predict an event variable. The Cox model estimates the hazard ratio, that is the ratio of the instantaneous probability of a given event occurring in a given time period comparing two groups. In Study I I, conditional Cox regression was used. This approach can be used when analysing matched data, that is when each case has one or more matched controls matched by certain variables by conducting the analysis in strata of the matching factors.
3.6.7 Matching
Matching is a statistical technique that is used in non-randomised studies to create two comparable groups with similar characteristics with respect to the matching variables.151 For each observation in the treatment group, one or more controls are assigned with the same values on the matching factors, for example age, sex, and BMI. Matching can be done with or without replacement. In matching with replacement the controls could be matched to multiple treatment observations, while in matching without replacement each control can only be matched to one treatment observation. 3.6.8 Meta-Analysis
A meta-analysis is a statistical analysis which combines the results of several independent studies.152
The statistical technique assigns different weight to the individual studies so that bigger and more
precise studies have more influence on the final summary value, compared to smal er less precise
studies. In the current thesis pooled risk ratios were assessed.
Two methods for estimating pooled effects from multiple studies are fixed and random effects meta-
analysis. The most common method is the random effects model when studies are identified from the
published literature. This method is used when it cannot be assumed that all studies are estimating the
same underlying value, which would be the case if al the included studies would be functional y
identical.
In the random effects model, two sources of variability are accounted for:
-
Within-Study Variability: This is the variability between subjects within a study (sampling error) Between-Study Variability: This is the variability between study effects in different studies (true variation in study effect sizes) When pooling studies in a meta-analysis, the presence of heterogeneity, the observed variability between study estimates, needs to be investigated and if it is large the source needs to be explored. Heterogeneity between studies is commonly assessed by the I2 statistic. If the I2 statistic exceeds 50%, it is recommended that the reason for heterogeneity among the studies should be investigated.153 Possible clinical sources of heterogeneity include treatment differences, such as doses or other medications given, or variation in included patients, such as sex, age, diseases.152
3.6.9 Missing Data
Although there are several different statistical methods for addressing missing data, none of these have been deemed sufficient replacements for measured data, and ultimately may generate bias in one way or the other. The most common approaches to handle missing data are: completers analysis, last-observation-carried forward, baseline observation carried forward and multiple imputation, al potential y resulting in different treatment effect estimates.154 In completers only analysis only participants completing the trial are included in the analysis. Hence an overestimation of the treatment effect is likely since those remaining in the trial are more likely to have been successful regarding their weight loss. Last observation carried forward includes the last measured value and will also, probably, lead to an overestimation of the treatment effect since the majority of the participants dropping out wil most likely gain weight after discontinuation. Baseline observation carried forward includes the baseline value of each missing value and therefore assumes that those who dropped out have returned to the baseline weight. This method is considered as the most conservative imputation approach. Multiple imputation is a method to impute missing values by using the association between observed characteristics and the outcome from values of the participants remaining in the study (and sometimes from those discontinuing using their values prior to discontinuation). Hence multiple imputation will often result in similar estimates as for the completers analysis. In the current thesis, baseline carried forward has been used as the main imputation method, while the other methods have been used in sensitivity analyses. 3.7 ROLE OF THE FUNDING SOURCES
Study I&I were partly supported by research grants from Cambridge Weight Plan, Northants, UK, and Novo Nordisk AS, Bagsværd, Denmark. The funders played no part in the analysis, write up of the papers, and did not read or comment on any version of the manuscript. Study I I was partly supported by a grant from Itrim International. The funders had no role in the design or conduct of the study; analysis or interpretation of the data; and did not read or comment on any version of the manuscript. Study IV was conducted without any specific funding. 4 RESULTS
4.1 STUDY I: WEIGHT LOSS AND OBSTRUCTIVE SLEEP APNOEA
Of 63 eligible patients, 30 were randomised to intervention and 33 to control. Two patients in the
control group were dissatisfied with al ocation and immediately discontinued. Al other patients
completed the randomised phase of the trial. Data from al randomised patients were included in an
intention-to-treat-analysis (baseline carried forward for missing data). Both groups had a mean AHI of
37 events/hour at baseline. At week nine, the difference between groups in AHI was -23 events/hour
(95%CI -15 to -30) and -20 kg (95%CI -18 to -21) in body weight, favouring the intervention group
(Table 15).
Table 15 Changes in weight and obstructive sleep apnoea (OSA) between baseline and week nine
difference
AHI (events/hour) -23 (-30 to -15) -20 (-21 to -18) Waist circumference (cm) -18 (-19 to -16) Neck circumference (cm) Data are mean (SD) or mean (95%CI); P values from independent sample t test; AHI=apnoea-hypopnoea index.
In the intervention group, 17% (5/30) were disease free (AHI <5) after the weight loss and 50% (15/30)
had mild disease (AHI 5-14.9), whereas AHI remained at 15 or greater in all controls indicating
moderate to severe disease (Figure 3). Significant treatment effect modification by baseline AHI was
found (P<0.001), with greater improvement in AHI in the intervention group among patients with
severe OSA (AHI >30) at baseline compared with those with moderate (AHI 15-30) sleep apnoea
(AHI -38 vs -12, P<0.001), despite similar weight loss (-19 vs -18 kg, P=0.55).
Figure 3 Proportions of patients defined as having no ("cured"), mild,
moderate, or severe obstructive sleep apnoea at week nine. Error
bars are 95% confidence intervals. Definitions: Cured defined as AHI <5 events/h, mild 5-14.9 events/h, moderate AHI 15-30 events/h, severe >30 events/h Adapted from Johansson, K et al (2009). "Effect of a very low energy diet on moderate and severe obstructive sleep apnoea in obese men: a randomised control ed trial." BMJ 339: b4609.
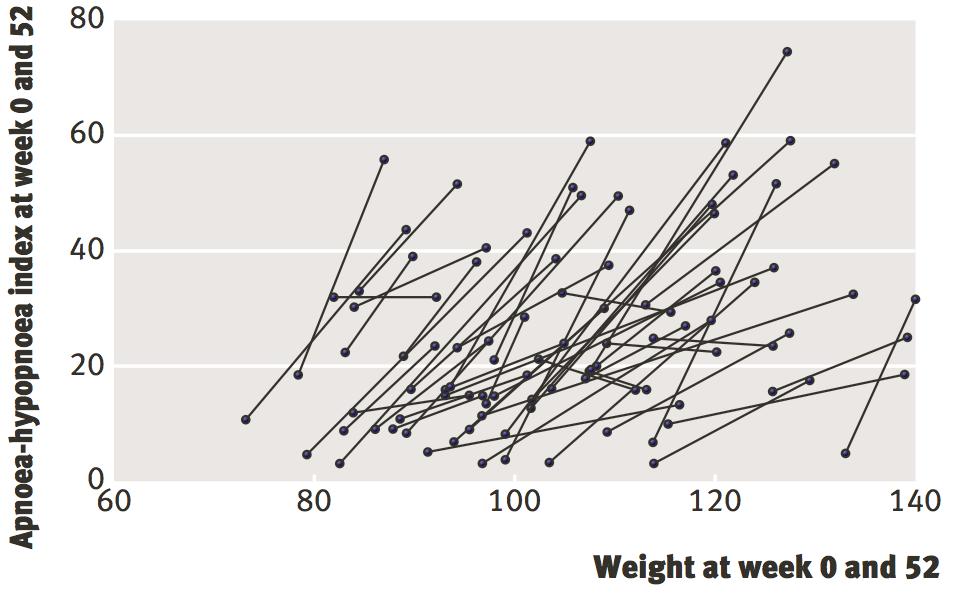
4.2 STUDY II: MAINTENANCE AND OBSTRUCTIVE SLEEP APNOEA
In the pooled observational study of maintenance of OSA improvements over one year, 58 of the 63
participants completed the VLED period and started the weight loss maintenance programme, with 44
completing the ful programme, and 49 with complete measurements at one year. In the main analysis,
data from al patients were analysed (baseline carried forward for missing data). At baseline mean AHI
was 36 events/hour, and mean weight was 113 kg. AHI changes after nine weeks of VLED were largely
maintained at one year (Table 16).
Table 16 Changes in weight and apnoea-hypopnoea index (AHI) during the one-year programme
Maintenance
Full programme
(0 to 9 weeks)
(>9 to 52 weeks)
(0 to 52 weeks)
Weight (kg)
BOCF (n=63)
Completers (n=44)
AHI (events/h)
BOCF (n=63)
Completers (n=44)
Data are mean (SD); P values from paired sample t test. BOCF=baseline carried forward for missing data.
AHI=apnoea-hypopnoea index.
After one year, patients with severe obstructive sleep apnoea at baseline had greater improvements in
AHI (-25 events/h; -54%) compared with patients with moderate disease (-7 events/h; -30%; P<0.001).
In addition, a dose-response association between weight loss and AHI at fol ow-up were seen (β=0.50
events/kg, P=0.01; Figure 4).
After one-year, 48% (30/63) no longer required CPAP during sleep and 10% (6/63) had achieved ful
remission (AHI<5).
Figure 4 Relation between AHI and
weight (kg) for al participants with measured weight and index at baseline
and follow-up (n=49). Lines indicate individuals. No participant gained weight, meaning that the right-most
observation represents the baseline value Adapted from Johansson, K et al (2011). "Longer
term effects of very low energy diet on obstructive
sleep apnoea in cohort derived from randomised control ed trial: prospective observational fol ow-up
study." BMJ 342: d3017.
During the VLED period, 13 patients had an adverse event classified as probably causally linked with the VLED. Of the 13 adverse events three were constipation, six were increased ALAT activity, one was dizziness, two were gouts and one was dry lips. Al adverse events had disappeared by the visit two weeks after the VLED period. During weight loss maintenance there were five additional adverse events of which three were gallstones (all three resulting in cholecystectomy), one was gout and one was kidney stones. No patient discontinued treatment because of adverse events.
4.3 STUDY III: RISK OF GALLSTONES DURING VLED
After matching LED participants (n=4,588) with replacement to VLED participants (n=3,773) 1:1 by
age, sex, BMI category, waist circumference category, and gallstone history, 3,320 participants
remained in each group. At baseline mean age, weight and BMI were 46 years, 95 kg, and 33 kg/m2,
respectively, while 83% were women. The majority were class I obese (51%), while 23% were class II
obese, 8% class III obese, and 19% overweight.
82% of VLED participants and 78% of LED participants completed the one-year programme. Weight
loss after one year was greater in the VLED than the LED group (-11 vs -8 kg, baseline carried forward
for missing data, and among completers -14 vs -11 kg, both P<0.001; Figure 5).
Figure 5 Change in weight during the one-year
Figure 6 Risk of gal stones requiring hospital care
treatment programmes
by weight loss programme
LOCF=last observation carried forward
VLED=very low energy diet
BOCF=baseline observation carried forward
LED=low energy diet
During 3,163 and 3,198 person-years in the VLED and LED groups, 48 and 14 gallstones requiring
hospital care occurred (152 vs 44 per 10,000 person-years; conditional hazard ratio 3.4, 95%CI 1.9
to6.1; P<0.001; Figure 6). The risk difference was 108 per 10,000 person-years (95%CI 59 to 157),
resulting in a number needed to harm of 92 (95%CI 63 to 168). That is, assuming a causal relationship,
one avoidable gallstone requiring hospital care would be caused for every 92 patients treated with
VLED instead of LED. Adjusting the analysis for weight loss during the first three months attenuated
the hazard ratio, but the risk remained statistical y significantly higher with VLED than LED (2.7, 95%CI
1.4 to 5.2).
Of the 62 participants with gallstones requiring hospital care, 39 (63%) resulted in cholecystectomy,
29 in the VLED and 10 in the LED group (conditional hazard ratio 3.1, 95%CI 1.5 to 6.5; P=0.003;
number needed to harm 151, 95%CI 94 to 377).
In a multivariable analysis including al subjects, the risk of developing gal stones requiring hospital care
was higher in women than men, in participants with a higher baseline BMI, among those who lost
≥10kg, and in those with a history of gallstones (irrespective of cholecystectomy status).
4.4 STUDY IV: TREATMENT DISCONTINUATION
A total of 28 randomised control ed trials met the inclusion criteria: 16 studies of orlistat (n=7,038), 7
of sibutramine (n=1,475) and 5 of rimonabant (n=4,944). All included studies were between 12 and 18
months duration. Patients had similar demographic profiles across trials of al three drugs, with
predominantly Caucasian subjects and a greater proportion of women than men in most of the studies.
The mean age ranged between 41 and 59 years and the mean BMI between 33 and 38 kg/m2.
The overall combined dropout rates in the included studies were high in drug and placebo arms, with
crude overal discontinuation rates of 30% for orlistat, 34% for sibutramine, 39% for rimonabant and
37% for placebo. In the overall pooled random effect model, the risk of dropout was marginal y but
statistical y significantly lower in the active drug compared to the placebo arms (pooled risk ratio 0.9;
95% 0.8 to 0.9; P=0.001; Figure 7).
Figure 7 Forest plot of the risk ratio (RR) of dropout from any cause in
the drug vs the placebo arms in rimonabant, sibutramine, orlistat and all
In the drug arms, the most common reasons for withdrawal were adverse events, patient request and poor compliance. In the placebo arms, withdrawal due to patient request and poor compliance were most common. Lack of effectiveness, as reason for dropout was specified in 10 studies. Patients in the drug arms tended to drop out less frequently as a result of lack of effectiveness compared with placebo (pooled risk ratio 0.5; 95% 0.4 to 0.7; P<0.001). The risk ratios for discontinuation due to adverse events were significantly elevated for rimonabant (2.0; 1.7-2.4) and orlistat (1.6; 1.2-2.1), but not sibutramine (1.0, 0.7-1.4), compared to placebo.
5 DISCUSSION
The overal objective of this thesis was to evaluate effects and side-effects of VLEDs in different obesity
settings, and to characterise discontinuation in obesity treatment. Specific objectives were to evaluate
weight loss as treatment option for patients with OSA (Study I&II); to assess the risk of gal stones
requiring hospital care in a commercial weight loss programme using VLED or LED (Study I I); and to
characterise overal discontinuation in obesity treatment programmes by analysing data from anti-
obesity drug trials (Study IV).
5.1 MAIN FINDINGS
The studies in this thesis were carried out at a specialist outpatient obesity centre (hospital-based;
Study I&II), or in the commercial sector (Study I I). The hospital-based trial included only men with OSA
(Study I&I ), while the commercial study (Study I I) included both men and women with the majority
being women. Analyses included al starting patients (baseline observation carried forward for missing
data).
Initial weight loss after VLED in the hospital-based treatment was -16% (Study I&I ), and -14% in the
commercial weight loss programme (Study I I). Hence the men in the hospital-based treatment
programme lost slightly more weight during VLED, but regained more during the maintenance phase
than the participants in the commercial programme (32% vs 19% regain of lost weight). A similar
weight loss at one year in the hospital-based (-11%) and commercial programme (-12%) was therefore
found.
Dropout from both the hospital-based and commercial programme was lower compared to the
benchmark of 37% in placebo arms of anti-obesity drugs trials (Study IV), with 30% in the hospital-based
and 18% in the commercial programme after one year. In the clinical care treatment programme,
gallstones were reported in 5% (3/63; al three cholecystectomised; mean BMI 35) of the participants
over one year compared to 1.4% (48/3320; 29 of 48 cholecystectomised; mean BMI 33) of the VLED
participants of the commercial weight loss programme.
5.1.1 Weight Loss and Obstructive Sleep Apnoea
The findings of the randomised trial of obese men with moderate to severe OSA (Study I) indicated that weight loss induced by a VLED significantly improved OSA. The mean AHI was reduced by two-thirds from 37 to 12 in the intervention group compared with no change in the weight stable control group after nine weeks. The pooled observational fol ow-up study (Study I ) showed that the AHI changes after nine weeks of VLED (-58%) were largely maintained at one year (-47%) fol owing the initial weight loss of -16% (-18kg), and -11% (-12kg) at one-year. In addition, a significant effect modification by baseline AHI was observed with patients with severe OSA at baseline having larger AHI improvements than patients with moderate disease. Moreover, a dose response association between weight loss and AHI improvement was found. After one year, half of the men no longer required CPAP, and one out of ten had total remission of OSA. There were also marked improvements in metabolic risk and the physical dimension of quality of life.
5.1.2 VLED and Risk of Gallstones
The absolute risk of gallstones requiring hospitalisation in overweight and obese participants enrol ed
in a one-year commercial weight loss programme (Study I I), using either VLED or LED during the initial
three-months weight loss phase, was found to be low (1.4% vs 0.4%). However, the risk was three
times higher in the VLED than the LED programme with a number needed to harm of 92. That is,
assuming a causal relationship between weight loss treatment and gallstone problems, one additional
gallstone requiring hospital care would result per 92 patients on VLED instead of LED. The
corresponding number for an additional cholecystectomy was 151. While the risks were greater in the
VLED compared to the LED group, the benefit in terms of one-year weight loss was also greater -12%
(-11 kg) vs -9% (-8 kg) of initial body weight, respectively. Also, a larger proportion of the participants
in the VLED treatment programme completed one-year of treatment compared to participants in LED
(82% vs 78%). These completion rates can be compared with the 63% in the placebo arms of
randomised trials of anti-obesity drugs.
5.1.3 Dropout in Anti-Obesity Drug Trials
Attrition in anti-obesity drug trials including overweight and obese participants was found to be high in
both the drug and placebo group, with overal discontinuation of 37% in the placebo arms only
receiving diet and lifestyle modification (Study IV), and 30-39% in the drug arms, although slightly lower
in the drug arm (pooled risk ratio 0.9). In the drug arms, the most common reasons for withdrawal
were adverse events, patient request and poor compliance. In the placebo arms, withdrawal due to
patient request and poor compliance were most common. Overal , patients in the drug arms tended to
drop out less frequently as a result of lack of effectiveness compared with placebo (pooled risk ratio
0.5). The risk ratios for discontinuation due to adverse events were significantly elevated for
rimonabant and orlistat, but not for sibutramine, compared to placebo.
5.2 COMPARISON WITH PREVIOUS RESEARCH
5.2.1 Weight Loss and Obstructive Sleep Apnoea
Before 2009, no randomised trial had shown weight loss to improve OSA, while observational studies of both dietary and surgical interventions indicated that this was the case.155 In 2009, three randomised control ed trials were published,114-116 one of which is included in this thesis (Study I).114 All three trials found a positive effect of weight loss on OSA, but in different patient groups. A Finnish study by Tuomilehto et al115 found a 40% reduction in AHI in patients with mild OSA after -11% (-11kg) weight loss at one year, and a 46% AHI reduction with a -7% (-7kg) weight loss after two years.140 In a sub-sample of the American Look AHEAD study, Foster et al116 found a 24% AHI reduction at one year with -10% (-11kg) weight loss after an intensive lifestyle intervention in older (mean age 61y) patients with type 2 diabetes, while the AHI increased in control patients, resulting in a between group AHI difference of 42%. The dose-response association that was found in Study I&II between weight loss and OSA was also found by Foster et al and et Tuomilehto al115 116 Foster et al also found significantly greater improvements in patients with severe disease at baseline, as found in Study I&II.
In addition to dietary interventions, weight loss induced by bariatric surgery has also been shown in
observational studies to result in long-term improvements in OSA. In the SOS study including matched
controls, Grunstein et al120 found that 30% of the surgery group reported self-reported persistent
sleep apnoea at the two-year fol ow-up compared with 70% of the control group. Smal uncontrol ed
studies (n<20) have also found long-term improvements in OSA after weight loss surgery.124 125 132 135 137
5.2.2 VLED and Risk of Gallstones
Few randomised trials investigating the effect of VLED on weight loss report side-effects. In a meta-analysis comparing the long-term efficacy and safety of VLEDs and LEDs, Tsai and Wadden65 concluded that no symptomatic gallstones were reported among VLED participants in any of the included trials, but they also concluded that this may have been attributable to lack of assessment in the individual studies. The majority of studies specifical y investigating the risk of gal stones during VLED treatment have been conducted in the late 1980s and early 1990s with VLEDs containing low levels of fat (≈1 g/day).90-94 In a review of these studies,88 Everhart reported that 10-25% of VLED participants developed gallstones, one third of which were symptomatic. However, the studies were of short duration (8-36 weeks),90-94 and only one study included a weight stable control group.92 Later studies have investigated VLEDs containing higher fat content,95-98 including two randomized control ed trials,95 96 and found that fewer participants developed symptomatic gallstones. In a review, Festi et al89 concluded that adequate fat content is important in gal stone prevention, with 10 g/day as a threshold for obtaining efficient gal bladder emptying. A lower recommendation of 7 g/day has, however, been given by the European SCOOP-report on VLED use.60 In our register-linkage study of a commercial weight loss programme using a liquid formula diet containing 7-9 g/day, we found a higher, albeit low, risk of symptomatic gallstones requiring hospitalisation in the VLED compared to the LED group, suggesting that more fat may be needed to eliminate the excess risk of gallstones compared to LED (Study I I). Bariatric surgery, however, also increases the risk of gal stones probably as a result of the rapid weight loss. The postoperative incidence has been reported to be between 3 to 28%.156 This large span may be explained by different fol ow-up time and/or different methods of detecting gal stones. A Swedish population-based cohort study of bariatric surgery identified the risk of gallstones requiring hospitalisation by the use of the National Patient Register (that is, similar to the methodology in Study III), and found a fivefold increased risk of both gal stones requiring hospital care and cholecystectomy for the bariatric surgery group compared to that of the general population. In the SOS study the incidence of gallstones and cholecystectomy after 2 years was assessed using questionnaires.157 Compared to conventional treatment, bariatric surgery significantly increased the risk of self-reported gallstones in men (4.0% vs 1.2%, odds ratio 4.2; P<0.001), but not in women (5.5% vs 4.5%, odds ratio 1.1; P=0.33). However, in both sexes, a greater weight loss in the surgery group was related to an increased incidence of gal stones.
5.2.3 Weight Loss Programme Discontinuation
Weight loss treatment programmes in observational settings (hospital-based and commercial) as wel
as randomised trials are generally associated with high levels of attrition, although reported dropout
rates vary considerably. In a systematic review of weight loss studies addressing factors associated with
attrition,142 one-year dropout rates of diet and lifestyle modification studies ranged between 16-77%. In
comparison, low attrition rates were reported in the Look AHEAD trial after 1 year (3% in the
intensive lifestyle intervention and 4% in the diabetes support and education intervention),24 and at four
years (6% vs 7%, respectively).25
Lack of treatment effect is one of the most common reasons why participants discontinue treatment.
In anti-obesity drug trials, adverse events are also a common reason in the active drug arm for
withdrawal, as reported in Study IV. Identified predictors of dropout in weight loss intervention studies
in general include young age, previous dieting attempts, too high weight loss expectations, poor mental
health, body dissatisfaction, low self-efficacy, low social support, and low initial weight loss.142
Attrition has been reported as the major limitation of al weight loss trials.158 Although there are
several different statistical methods for addressing missing data, none of these have been deemed
sufficient replacement for measured data, and ultimately generate bias in one way or the other.154
When interpreting estimates of weight loss interventions it is important to consider how missing data
have been handled, especially when comparing treatment effects of different studies.
5.3 STRENGTHS AND LIMITATIONS
Study Design: Study I was a randomised control ed trial of nine week duration, fol owed by an
observational study of a total duration of one year (Study I ). The strengths of Study I was the
randomised study design with its low probability of selection bias and residual confounding.159 The
reason for not using a randomised design with a one-year fol ow-up was that our primary aim was to
show, in the short term, a treatment effect from weight loss with a VLED in moderate to severe OSA.
Our secondary aim was to see whether any improvements could be maintained in the long-term. We
therefore wanted to minimise non-compliance in the control group in the nine week randomised phase
by providing a strong incentive for controls to remain in the study by al owing patients to start the
VLED programme immediately after serving as controls. The limitation of the observational design
used for the one-year follow up means that our analysis is limited by the lack of comparison with
natural progression regarding AHI and weight.
Study I I was an observational cohort study with a large sample of participants in a commercial weight
loss programme in a real-life setting. Because the risk of gal stones is low, one strength was the large
sample which provided sufficient power for a comparison with LED. Also, by using a register-linkage
design for assessment of events, we could fol ow-up a large number of participants at a relatively low
cost. The main limitation was that participants were not randomised, but had self-selected to the VLED
and LED programmes. However, the ensuing baseline differences in age, sex, BMI, waist circumference,
and gal stone history were handled by performing a matched analysis. This design reduces the
likelihood and impact of confounding, but residual confounding may remain beyond the matching
factors.
Study IV was a meta-analysis which combined results from individual anti-obesity drug trials and
assessed the risk of treatment discontinuation. The strengths of the meta-analysis include: increased
statistical power, improved estimates of effect size and the ability to resolve controversies when
different studies report conflicting results regarding both overal discontinuation and discontinuation
due to adverse events.
Identification/measurement of main outcomes: In Study I&II the main outcome measure was
AHI derived from two consecutive nocturnal sleep studies in the home. Because of the reported night-
to-night variability,160 a strength was that duplicate sleep studies were performed. A limitation was the
use of a portable WatchPAT device instead of polysomnography, which is considered the gold
standard for diagnosing OSA. However, high costs, limited access to sleep laboratories, and the
increasing number of patients with OSA have led to the development of more accessible and cheaper
methods, such as the portable monitors used in this study. Validation studies show that the WatchPAT
device has high sensitivity and specificity for estimating sleep time and AHI compared with
polysomnography.161 162
In Study I I, the strength was the use of nationwide register data to identify the main outcome
(gal stones requiring hospital care), the secondary outcome (cholecystectomy), and co-morbidity
history and drug use of the cohort. The National Patient Register and the Prescribed Drug Register
contain prospectively reported data col ected routinely on a nationwide level in the universally
accessible Swedish health care system, with virtually complete fol ow-up. One limitation, however, is
that the National Patient Register only includes data on inpatient and non-primary outpatient visits and
not visits in primary care. Hence, symptomatic gallstones treated in primary care could not be
identified. However, our primary outcome was gallstones requiring hospital care, since symptomatic
gallstones treated in primary care, at home, or not at al , are likely to be both less serious for the
patient and less costly for society.
Study Populations: Study I&II included a patient group reported to have an increased risk for
mortality.110-112 However, since we only included obese men aged 30-65 years, our results might not be
generalisable to subjects outside the inclusion criteria of the study, that is women, younger (<30 years)
or older (>65 years) patients, overweight (BMI 25-29.9) or extremely obese patients (BMI ≥40), or
patients with mild sleep apnoea. The rationale for only including men was mainly to reduce risk of type
2 error, since we could not be certain that the effect of weight loss on OSA would be the same for
men and women. The reason for choosing men instead of women was that the prevalence of OSA is
much higher in men vs women (24% vs 9%, respectively).99 The strengths of Study I I included a large
sample of weight loss participants in a real-life setting. The results from this study may limit
generalisability to the wider overweight and obese population, since the participants had selected and
paid for the treatment themselves, and could therefore be considered as a highly motivated group of
higher socio-economic class.
5.4 CLINICAL IMPLICATIONS
The availability of obesity treatment is today limited mainly to lifestyle modification or bariatric surgery, since the withdrawal of two out of three previously approved anti-obesity drugs. Bariatric surgery is currently the most effective method for treating obesity and results in weight loss of about 14-25% after 10 years.52 However, it is not realistic to treat al obese patients with surgery. According to current guidelines, only class II and class III obese qualify, that is patients with a BMI >35. Furthermore, due to both presence of contraindications and capacity constraints, not al in the class II/III obese group would be operated upon either. Other non-surgical treatment options, with strict maintenance programmes, are therefore needed. VLED in combination with a strict maintenance programme has been found to be a strong non-surgical candidate for achieving one-year maintenance results of 10-15% weight loss.33 72 74-81
The long-term effects of the use of VLED in the treatment of obesity vs LED have however been questioned. In the meta-analysis of Tsai and Wadden, they concluded that VLEDs induced significantly greater short-term weight loss than LEDs (mean difference of initial weight loss -6.4%, p<0.001) during a mean initial 13 weeks, but similar losses after 2 years (-1.3%, p>0.02).65 They also concluded that VLEDs would be more attractive if sufficient weight loss maintenance programmes existed, but they did not recommend the use of VLEDs over LEDs. However, neither of the included studies of that meta-analysis included an extensive exercise programme,74 75 pharmaco-therapy,76-81 a prolonged re-feeding period72 75 or a low glycemic index diet in combination with high protein33 as a part of the maintenance programme, which have all been proven to limit weight regain or even reduce weight further. 33 74 76 80 81
In the VLED group of Study I I 81% of the initial weight loss was maintained up to one-year (98% among completers), although the adjusted mean difference between VLED and LED was reduced from -5 kg at 12 weeks to -3kg at one-year. In Study I&II 68% (82% among completers) of initial weight was maintained up to one-year. The larger maintained weight loss in Study I I could be explained by regular scheduled exercise as compared to Study II where increased exercise was emphasised but the programme did not include an exercise scheme. Also, Study I I included a highly motivated group that had sought and also paid for treatment themselves.
Regarding the safety aspects of VLED, as shown in Study I I, there is an increased risk of symptomatic gallstones requiring hospital care as well as cholecystectomy when using VLED instead of LED, although the absolute risk was low. Increased risk for gal stones is, however, also seen after bariatric surgery, which currently is considered the most effective treatment for obesity.
Whether the benefits of the additional weight loss in the VLED group compared to the LED group are worth the extra risk for gal stones and cholecystectomy, as well as the extra health care costs, may depend on patients' disease and risk factor status as well as their preferences. For some patients, VLED could however be difficult to adhere to and/or result in significant weight regain after the weight loss phase, while others will benefit from VLEDs both in the short and long-term. Although little is known about which patients are most likely to benefit from different treatments, a study by Gripeteg et al163 has investigated predictors of VLED outcome. The authors found that social support and walking capacity were important determinants of successful weight loss with VLED in men, whereas psycho-social function were important for VLED success in women. They also found that patients with low perceived health who lack a close social network may need extra support during treatment.
5.5 FUTURE RESEARCH
Weight loss maintenance after VLED: Further long-term randomised studies, including
comparison of the effect of different weight loss maintenance strategies after VLED or LED are
needed to optimise weight loss maintenance.
Composition of VLED: The effect of different fat contents of VLEDs has not been studied in detail
in long-term studies. Hence comparisons between products containing about 7 g/day of fat as
recommended by the European SCOOP-report on VLED use, and higher intakes (>12 g/day) are
needed to evaluate associated risk for gallstones with a lower fat intake of VLEDs.
Long-term effect of weight loss on OSA: Weight loss may have a long-term effect on OSA
despite a rebound in weight. Tuomilehto et al140 demonstrated in their two-year fol ow-up study
that AHI remained improved and did not relapse and fol ow the trend of modest weight gain. Also,
unpublished data of the four-year fol ow-up of the study by Foster et al116 demonstrated the same
trend. Further long-term studies of the effect of weight loss in OSA are therefore needed to
investigate this long-term response to weight loss.
Prevention of discontinuation: The majority of published weight loss studies focuses on the
effects of weight loss and/or the resolution of obesity-related co-morbidities, and reasons for
dropout are often either not reported, or not explored in any depth. The few studies that have
investigated reasons for dropout have been limited to data not col ected for the purpose of
evaluation of dropout.142 Studies are therefore needed to identify strategies to prevent dropout.
6 CONCLUSION
The overal conclusions of this thesis are:
I) VLED-induced weight loss resulted in a significant reduction of moderate to severe OSA in obese
men. Patients with severe OSA benefited most from the VLED-induced weight loss.
II) In patients with moderate to severe OSA who had lost weight by a VLED, the majority of the
initial improvement in AHI was maintained at one year. Almost half of the patients no longer required CPAP after one year, and one out of ten had total remission of OSA.
III) Although VLED, compared to LED, is associated with a three times increased risk of gallstones
requiring hospital care or cholecystectomy, the absolute risk was low (1.4% for VLED vs 0.4% for LED). While these risks were greater for VLED compared to LED, sustained weight loss reduction was approximately 30% greater at one year in the VLED group.
IV) One-year treatment discontinuation was lower in both the sleep apnoea study using a hospital-
based outpatient weight loss programme (30%) and in the commercial weight loss programme (18%) compared to the pooled data from placebo arms of anti-obesity drug trials (37%).
7 SVENSK SAMMANFATTNING
Bakgrund Förekomsten av fetma har ökat dramatiskt under de senaste decennierna, både i Sverige
och i övriga världen. Fetma är associerat med ökad risk för sjuklighet och dödlighet, vilket leder til stor
belastning för sjukvården. Den för närvarande mest effektiva fetmabehandlingen är fetmakirurgi.
Eftersom al a individer med fetma inte kan genomgå fetmakirurgi är behovet av icke-kirurgiska
behandlingsmetoder stort.
Syfte Det övergripande syftet med denna avhandling var att utvärdera effekter och bieffekter av "very
low energy diets" (VLEDs), men också att karakterisera avhopp från fetmabehandling. Specifika syften
var att utvärdera effekten av viktminskning med VLED som behandling för patienter med obstruktiv
sömnapné (OSA; Studie I&II) i ett kliniskt viktminskningsprogram, att utvärdera risken för sjukvårds-
krävande gallstensbesvär efter VLED el er "low energy" diet (LED, Studie III) i ett kommersiel t
viktminskningsprogram, och att karakterisera avhopp från fetmabehandling genom att analysera data
från fetmaläkemedelsprövningar (Studie IV).
Metoder Studie I&II bestod av en nio veckor lång randomiserad kontrol erad studie följd av 43
veckors observationel uppföljning. Män med fetma (n=63, BMI 30-40, 30-65 år) och måttlig til svår
OSA (apnea-hypopnea index (AHI) ≥ 15) behandlade med CPAP inkluderades. Interventionen bestod
av VLED (554 kcal/dag) under nio veckor. Efter den randomiserade fasen erhöl kontrol gruppen
samma intervention. VLED-perioden följdes av sedan av ett viktstabiliseringsprogram. Studie III var en
ettårig matchad kohortstudie, bestående av män och kvinnor som deltog i ett kommersiel t
viktminskningsprogram i Sverige mellan 2006 och 2009 (n=6,640; medelålder 46 år, 83% kvinnor, BMI
33). Programmet inkluderade en tremånaders viktminskningsperiod bestående antingen av VLED (500
kcal/dag) el er LED (1,200-1,500 kcal/dag). Viktminskningsfasen följdes av ett nio månaders
viktstabiliseringsprogram. Data om sjukvårdskrävande gal stensbesvär eller kolecystektomi under det
ettåriga programmet hämtades från patientregistret på Socialstyrelsen. Studie IV var en systematisk
genomgång och metaanalys som inkluderade publicerade randomiserade placebokontrol erade
läkemedelsprövningar av orlistat, sibutramin och rimonabant (n=13,457).
Resultat Studie I&II: Efter den nio veckor långa randomiserade fasen var interventionsgruppens
genomsnittliga kroppsvikt 20 kg lägre än kontrollgruppens, och medelvärdet för AHI var 23
uppehål /timme lägre. Totalt så fullföljde 70% (44/63) av al a deltagare den observationel a uppföljnings-
studien. Merparten av de initiala förbättringarna i AHI (-58%) efter ett år bibehöl s (-47%) efter den
initiala viktminskningen på 18 och 12 kg efter ett år. Studie III: Den absoluta risken för gallsten och
kolecystektomi var låg, men tre gånger högre i VLED än LED programmet (hasardkvot 3.4 och 3.1;
P<0.001). Medan riskerna för gal sten var större i VLED- jämfört med LED-gruppen så var
viktminskningen också större (11 vs 8 kg; P<0.001) och fler fullföljde studien (82% vs 78%). Studie IV:
Avhoppsfrekvensen från fetmaläkemedelsprövningar var hög både i läkemedels- (30-39%) och
kontrol grupperna (37%) men något lägre i läkemedelsgruppen (poolad riskkvot 0.9; P=0.001).
Slutsats VLED-inducerad viktnedgång resulterade i en signifikant reduktion av måttlig til svår OSA,
med majoriteten av den initiala förbättringen bibehål en efter ett år. Risken för gallsten var högre efter
VLED än LED, medan viktminskningen också var större i VLED-gruppen. Frekvensen behandlings-
avhopp var lägre i både det kliniska och det kommersiella viktminskningsprogrammet jämfört med
poolade data från kontrol grupperna i fetmaläkemedelsprövningar.
8 ACKNOWLEDGMENT
I would like to thank,
My supervisor, Martin Neovius, for everything you have taught me; for always being available regardless
the time of the day (and night); for the uncountable hours and endless commitment; for always pushing
me and for never letting me give up. Simply lots of thanks for being a fantastic main supervisor.
Erik Hemmingsson, co-supervisor, for al your support and encouragement; for never being further away
than a phone cal and for al the inspiring discussions.
Stephan Rössner, co-supervisor, for always believing in me; for always saying your opinion; for your
extremely rapid response in proof-reading; for making the OSA-study happen; and finally for your
humour and fun discussions.
Finally to al my supervisors for believing in me and al owing me to take responsibility; for placing high
expectations on me while at the same time giving me al the help I ever needed. Thank you for four
wonderful years.
Kristian Neovius for sharing the PhD-student time with me; for being a fantastic friend ever since we
shared office at the Obesity Unit; for al the fun discussions; for al your help and support with
everything from KI-forms to bicycle repairs to cooking.
Anna Laumann, Jenny Dygve and Sara Yl ö, for the fantastic years at the Obesity Unit; for all the laughter;
for showing the importance of having a calendar; for putting up with me when I did not have the time
for coffee breaks or anything else; for making work a wonderful place; and finally for being fantastic
friends ever since that time.
Lena Mannström for all the lovely discussions; for always having the time for me and helping me when
everything was new; for giving me a hand with everything from paper jams to study-planning and of
course for doing an excel ent job in the OSA-study.
Jonas Eriksson for al your SAS support. It is a true honour to share office with a human SAS-
encyclopaedia; and for al the inspiring cross-country skiing discussions.
Viveca Petré for help with everything from administration to study-planning and for al the nice chats
during the years at the Obesity Unit.
Mary Hyl for al the interesting discussions and for excellent proof-reading of manuscripts.
Richard Harlid for great collaboration in the OSA-study; for al your hard work with everything from
patient inclusion to manually reviewing al the sleep data; for delivering the data under time pressure;
and final y for all your help, guidance and patience with al my questions about sleep apnoea.
Ylva Trol e Lagerros for interesting research discussions and help with the OSA-study.
Al col eagues at the Obesity Unit, Huddinge, for making the time so fun and educational. A special thanks to Lena Mannström, Jenny Dygve, Mary Hyl , Ylva Trol e Lagerros, Viveca Petré, Anna Laumann and Sara Yl ö for your hard, dedicated and thorough work in the OSA-study. The staff at Aleris Fysiologlab, for performing the sleep studies. The study patients in the OSA-study for participating in the study. Fredrik Granath for statistical expertise. Birgitta Thörn, Itrim, for al the work with the data extraction in the commercial weight loss study. Johan Sundström and Claude Marcus for co-authorship, great comments and new ideas. Friends and Family, To Johanna, my oldest friend, for making me somewhat more spontaneous and for your support throughout al years. Astrid, my "big sister", for always guiding me and helping me see things from new perspectives; for your endless encouragement and support; and for always giving me your honest opinion. Anna for always being there for me; always supporting me; and for always reminding me to take care of myself when I become absorbed in work or other projects. Last but not least, to my family, to Björn for your patience, endless support and encouragement; for always believing in me and my abilities; and for putting up with al my ideas and projects. To my parents, Agneta and Svante, for always encouraging me to do what I believe in and for your everlasting support.
9 REFERENCES
1. WHO. Obesity: preventing and managing the global epidemic: report of a WHO consultation. Geneva:
World Health Organization, 2000.
2. Pischon T, Boeing H, Hoffmann K, Bergmann M, Schulze MB, Overvad K, et al. General and
abdominal adiposity and risk of death in Europe. The New England journal of medicine
2008;359(20):2105-20.
3. Alberti KG, Eckel RH, Grundy SM, Zimmet PZ, Cleeman JI, Donato KA, et al. Harmonizing the
metabolic syndrome: a joint interim statement of the International Diabetes Federation Task
Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American
Heart Association; World Heart Federation; International Atherosclerosis Society; and
International Association for the Study of Obesity. Circulation 2009;120(16):1640-5.
4. Finucane MM, Stevens GA, Cowan MJ, Danaei G, Lin JK, Paciorek CJ, et al. National, regional, and
global trends in body-mass index since 1980: systematic analysis of health examination surveys
and epidemiological studies with 960 country-years and 9.1 mil ion participants. Lancet
2011;377(9765):557-67.
5. Flegal KM, Carrol MD, Kit BK, Ogden CL. Prevalence of obesity and trends in the distribution of
body mass index among US adults, 1999-2010. JAMA : the journal of the American Medical
Association 2012;307(5):491-7.
6. Statistiska-centralbyrån. Levnadsförhållanden - Hälsa, sjukdom och vård efter region, indikator och
kön. Andelar i procent och skattat antal i tusental. 3-årsmedelvärde. Fetma och övervikt. 2008-
Accessed 2012-03-30.
7. Gorber SC, Tremblay M, Moher D, Gorber B. A comparison of direct vs. self-report measures for
assessing height, weight and body mass index: a systematic review. Obes Rev 2007;8(4):307-26.
8. Sturm R. Increases in morbid obesity in the USA: 2000-2005. Public Health 2007;121(7):492-6.
9. Neovius M, Teixeira-Pinto A, Rasmussen F. Shift in the composition of obesity in young adult men in
Sweden over a third of a century. Int J Obes (Lond) 2008;32(5):832-6.
10. Flegal KM, Carrol MD, Ogden CL, Curtin LR. Prevalence and trends in obesity among US adults,
1999-2008. JAMA : the journal of the American Medical Association 2010;303(3):235-41.
11. Rokholm B, Baker JL, Sorensen TI. The levelling off of the obesity epidemic since the year 1999--a
review of evidence and perspectives. Obes Rev 2010;11(12):835-46.
12. Haslam DW, James WP. Obesity. Lancet 2005;366(9492):1197-209.
13. Neovius K, Johansson K, Kark M, Neovius M. Obesity status and sick leave: a systematic review.
Obes Rev 2009;10(1):17-27.
14. Neovius K, Johansson K, Rossner S, Neovius M. Disability pension, employment and obesity status:
a systematic review. Obes Rev 2008;9(6):572-81.
15. Neovius K, Neovius M, Rasmussen F. The combined effects of overweight and smoking in late
adolescence on subsequent disability pension: a nationwide cohort study. Int J Obes (Lond)
2010;34(1):75-82.
16. Withrow D, Alter DA. The economic burden of obesity worldwide: a systematic review of the
direct costs of obesity. Obes Rev 2011;12(2):131-41.
17. Odegaard K, Borg S, Persson U, Svensson M. The Swedish cost burden of overweight and obesity--
evaluated with the PAR approach and a statistical model ing approach. Int J Pediatr Obes 2008;3
18. Whitlock G, Lewington S, Sherliker P, Clarke R, Emberson J, Halsey J, et al. Body-mass index and
cause-specific mortality in 900 000 adults: col aborative analyses of 57 prospective studies.
Lancet 2009;373(9669):1083-96.
19. Neovius M, Sundstrom J, Rasmussen F. Combined effects of overweight and smoking in late
adolescence on subsequent mortality: nationwide cohort study. BMJ 2009;338:b496.
20. McGee DL. Body mass index and mortality: a meta-analysis based on person-level data from
twenty-six observational studies. Ann Epidemiol 2005;15(2):87-97.
21. Flegal KM, Graubard BI, Wil iamson DF, Gail MH. Excess deaths associated with underweight,
overweight, and obesity. JAMA : the journal of the American Medical Association
2005;293(15):1861-7.
22. Mokdad AH, Marks JS, Stroup DF, Gerberding JL. Actual causes of death in the United States, 2000.
JAMA : the journal of the American Medical Association 2004;291(10):1238-45.
23. Franz MJ, VanWormer JJ, Crain AL, Boucher JL, Histon T, Caplan W, et al. Weight-loss outcomes:
a systematic review and meta-analysis of weight-loss clinical trials with a minimum 1-year
fol ow-up. J Am Diet Assoc 2007;107(10):1755-67.
24. Pi-Sunyer X, Blackburn G, Brancati FL, Bray GA, Bright R, Clark JM, et al. Reduction in weight and
cardiovascular disease risk factors in individuals with type 2 diabetes: one-year results of the
look AHEAD trial. Diabetes care 2007;30(6):1374-83.
25. Wing RR. Long-term ef ects of a lifestyle intervention on weight and cardiovascular risk factors in
individuals with type 2 diabetes mel itus: four-year results of the Look AHEAD trial. Arch Intern
Med 2010;170(17):1566-75.
26. Lindroos A-K, Rössner S. Fetma : från gen- til samhällspåverkan. Lund: Studentlitteratur, 2007.
27. Nordmann AJ, Nordmann A, Briel M, Kel er U, Yancy WS, Jr., Brehm BJ, et al. Effects of low-
carbohydrate vs low-fat diets on weight loss and cardiovascular risk factors: a meta-analysis of
randomized control ed trials. Arch Intern Med 2006;166(3):285-93.
28. Astrup A, Meinert Larsen T, Harper A. Atkins and other low-carbohydrate diets: hoax or an
effective tool for weight loss? Lancet 2004;364(9437):897-9.
29. Foster GD, Wyatt HR, Hil JO, Makris AP, Rosenbaum DL, Brill C, et al. Weight and metabolic
outcomes after 2 years on a low-carbohydrate versus low-fat diet: a randomized trial. Ann
Intern Med 2010;153(3):147-57.
30. Shai I, Schwarzfuchs D, Henkin Y, Shahar DR, Witkow S, Greenberg I, et al. Weight loss with a
low-carbohydrate, Mediterranean, or low-fat diet. The New England journal of medicine
2008;359(3):229-41.
31. Sacks FM, Bray GA, Carey VJ, Smith SR, Ryan DH, Anton SD, et al. Comparison of weight-loss
diets with different compositions of fat, protein, and carbohydrates. The New England journal of
medicine 2009;360(9):859-73.
32. Wing RR, Phelan S. Long-term weight loss maintenance. Am J Clin Nutr 2005;82(1 Suppl):222S-25S.
33. Larsen TM, Dalskov SM, van Baak M, Jebb SA, Papadaki A, Pfeiffer AF, et al. Diets with high or low
protein content and glycemic index for weight-loss maintenance. The New England journal of
medicine 2010;363(22):2102-13.
34. Wing RR, Hil JO. Successful weight loss maintenance. Annual review of nutrition 2001;21:323-41.
35. Wadden TA, Neiberg RH, Wing RR, Clark JM, Delahanty LM, Hill JO, et al. Four-year weight losses
in the Look AHEAD study: factors associated with long-term success. Obesity (Silver Spring)
2011;19(10):1987-98.
36. Wing RR, Tate DF, Gorin AA, Raynor HA, Fava JL. A self-regulation program for maintenance of
weight loss. The New England journal of medicine 2006;355(15):1563-71.
37. Tsai AG, Wadden TA. Systematic review: an evaluation of major commercial weight loss programs
in the United States. Ann Intern Med 2005;142(1):56-66.
38. Heshka S, Anderson JW, Atkinson RL, Greenway FL, Hill JO, Phinney SD, et al. Weight loss with
self-help compared with a structured commercial program: a randomized trial. JAMA : the
journal of the American Medical Association 2003;289(14):1792-8.
39. Truby H, Baic S, deLooy A, Fox KR, Livingstone MB, Logan CM, et al. Randomised control ed trial
of four commercial weight loss programmes in the UK: initial findings from the BBC "diet
trials". BMJ 2006;332(7553):1309-14.
40. Dansinger ML, Gleason JA, Griffith JL, Selker HP, Schaefer EJ. Comparison of the Atkins, Ornish,
Weight Watchers, and Zone diets for weight loss and heart disease risk reduction: a
randomized trial. JAMA : the journal of the American Medical Association 2005;293(1):43-53.
41. Jebb SA, Ahern AL, Olson AD, Aston LM, Holzapfel C, Stol J, et al. Primary care referral to a
commercial provider for weight loss treatment versus standard care: a randomised control ed
trial. Lancet 2011;378(9801):1485-92.
42. Padwal RS, Majumdar SR. Drug treatments for obesity: orlistat, sibutramine, and rimonabant. Lancet
2007;369(9555):71-7.
43. Rucker D, Padwal R, Li SK, Curioni C, Lau DC. Long term pharmacotherapy for obesity and
overweight: updated meta-analysis. BMJ 2007;335(7631):1194-9.
44. Heal DJ, Gosden J, Smith SL. What is the prognosis for new central y-acting anti-obesity drugs?
Neuropharmacology 2012.
45. Johansson K, Sundstrom J, Neovius K, Rossner S, Neovius M. Long-term changes in blood pressure
fol owing orlistat and sibutramine treatment: a meta-analysis. Obes Rev 2010;11(11):777-91.
46. Christensen R, Kristensen PK, Bartels EM, Bliddal H, Astrup A. Efficacy and safety of the weight-
loss drug rimonabant: a meta-analysis of randomised trials. Lancet 2007;370(9600):1706-13.
47. James WP, Caterson ID, Coutinho W, Finer N, Van Gaal LF, Maggioni AP, et al. Effect of
sibutramine on cardiovascular outcomes in overweight and obese subjects. The New England
journal of medicine 2010;363(10):905-17.
48. EMEA. European Medicines Agency: European Medicines Agency recommends first switch from
prescription only to non-prescription for a central y authorised medicine. 2008;
Accessed 2012-03-30.
49. Astrup A, Carraro R, Finer N, Harper A, Kunesova M, Lean ME, et al. Safety, tolerability and
sustained weight loss over 2 years with the once-daily human GLP-1 analog, liraglutide. Int J
Obes (Lond) 2011.
50. Dixon JB, Straznicky NE, Lambert EA, Schlaich MP, Lambert GW. Surgical approaches to the
treatment of obesity. Nature reviews. Gastroenterology & hepatology 2011;8(8):429-37.
51. Nationel a-indikationer-för-obesitas-kirurgi. 2009;
Accessed 2012-03-30.
52. Sjostrom L, Narbro K, Sjostrom CD, Karason K, Larsson B, Wedel H, et al. Effects of bariatric
surgery on mortality in Swedish obese subjects. The New England journal of medicine
2007;357(8):741-52.
53. Colquitt JL, Picot J, Loveman E, Clegg AJ. Surgery for obesity. Cochrane Database Syst Rev
2009(2):CD003641.
54. Buchwald H, Avidor Y, Braunwald E, Jensen MD, Pories W, Fahrbach K, et al. Bariatric surgery: a
systematic review and meta-analysis. JAMA : the journal of the American Medical Association
2004;292(14):1724-37.
55. Sjostrom L, Lindroos AK, Peltonen M, Torgerson J, Bouchard C, Carlsson B, et al. Lifestyle,
diabetes, and cardiovascular risk factors 10 years after bariatric surgery. N Engl J Med
2004;351(26):2683-93.
56. Sjostrom L, Peltonen M, Jacobson P, Sjostrom CD, Karason K, Wedel H, et al. Bariatric surgery
and long-term cardiovascular events. JAMA : the journal of the American Medical Association
2012;307(1):56-65.
57. Karlsson J, Taft C, Ryden A, Sjostrom L, Sul ivan M. Ten-year trends in health-related quality of life
after surgical and conventional treatment for severe obesity: the SOS intervention study. Int J
Obes (Lond) 2007;31(8):1248-61.
58. Sjostrom L, Gummesson A, Sjostrom CD, Narbro K, Peltonen M, Wedel H, et al. Effects of
bariatric surgery on cancer incidence in obese patients in Sweden (Swedish Obese Subjects
Study): a prospective, control ed intervention trial. The lancet oncology 2009;10(7):653-62.
59. Näslund I. Accelererande utveckling av obesitaskirurgi i Sverige. Lakartidningen 2011;108(49):2574-
60. SCOOP-VLCD Task 7.3. Scientific Co-operation on Questions Relating to Food: Directorate-
Accessed 2012-03-20.
61. National Task Force on the Prevention and Treatment of Obesity NIoH. Very low-calorie diets.
JAMA : the journal of the American Medical Association 1993;270(8):967-74.
62. National Institutes of Health NIoH. Clinical Guidelines on the Identification, Evaluation, and
Treatment of Overweight and Obesity in Adults--The Evidence Report. National Institutes of
Health. Obes Res 1998;6 Suppl 2:51S-209S.
63. NIH. Obesity guidance on the prevention, identification, assessment and management of
overweight and obesity in adults and children (NICE Clinical Guideline 43). 2006:4S-80S.
64. Sharma AM, Freedhoff Y. Best Weight- Practical Guide to Office-Based Obesity Management: Canadian
Obesity Network 2010.
65. Tsai AG, Wadden TA. The evolution of very-low-calorie diets: an update and meta-analysis. Obesity
(Silver Spring) 2006;14(8):1283-93.
66. Mustajoki P, Pekkarinen T. Very low energy diets in the treatment of obesity. Obes Rev
2001;2(1):61-72.
67. Codex Stan 203-1995. Codex Standard for formula foods for use in very low energy diets for
weight reduction. 1995;
Accessed 2012-03-20.
68. Torgerson JS, Agren L, Sjostrom L. Effects on body weight of strict or liberal adherence to an initial
period of VLCD treatment. A randomised, one-year clinical trial of obese subjects. Int J Obes
Relat Metab Disord 1999;23(2):190-7.
69. Heymsfield SB, van Mierlo CA, van der Knaap HC, Heo M, Frier HI. Weight management using a
meal replacement strategy: meta and pooling analysis from six studies. Int J Obes Relat Metab
Disord 2003;27(5):537-49.
70. Torgerson JS. Very Low Calorie Diets In: Lindroos A-K, Rössner S, editors. Fetma : från gen- til
samhäl spåverkan. Lund: Studentlitteratur, 2007:253-64.
71. Van Nieuwenhove Y, Dambrauskas Z, Campil o-Soto A, van Dielen F, Wiezer R, Janssen I, et al.
Preoperative very low-calorie diet and operative outcome after laparoscopic gastric bypass: a
randomized multicenter study. Arch Surg 2011;146(11):1300-5.
72. Gripeteg L, Torgerson J, Karlsson J, Lindroos AK. Prolonged refeeding improves weight
maintenance after weight loss with very-low-energy diets. The British journal of nutrition
2010;103(1):141-8.
73. Atkinson RL. Low and very low calorie diets. The Medical clinics of North America 1989;73(1):203-15.
74. Fogelholm M, Kukkonen-Harjula K, Nenonen A, Pasanen M. Effects of walking training on weight
maintenance after a very-low-energy diet in premenopausal obese women: a randomized
control ed trial. Arch Intern Med 2000;160(14):2177-84.
75. Bischoff SC, Damms-Machado A, Betz C, Herpertz S, Legenbauer T, Low T, et al. Multicenter
evaluation of an interdisciplinary 52-week weight loss program for obesity with regard to body
weight, comorbidities and quality of life-a prospective study. Int J Obes (Lond) 2011.
76. Apfelbaum M, Vague P, Ziegler O, Hanotin C, Thomas F, Leutenegger E. Long-term maintenance of
weight loss after a very-low-calorie diet: a randomized blinded trial of the efficacy and
tolerability of sibutramine. The American journal of medicine 1999;106(2):179-84.
77. LeCheminant JD, Jacobsen DJ, Hall MA, Donnelly JE. A comparison of meal replacements and
medication in weight maintenance after weight loss. Journal of the American Col ege of Nutrition
2005;24(5):347-53.
78. Mathus-Vliegen EM. Long-term maintenance of weight loss with sibutramine in a GP setting
fol owing a specialist guided very-low-calorie diet: a double-blind, placebo-controlled, paral el
group study. European journal of clinical nutrition 2005;59 Suppl 1:S31-8; discussion S39.
79. Richelsen B, Tonstad S, Rossner S, Toubro S, Niskanen L, Madsbad S, et al. Effect of orlistat on
weight regain and cardiovascular risk factors fol owing a very-low-energy diet in abdominal y
obese patients: a 3-year randomized, placebo-control ed study. Diabetes care 2007;30(1):27-32.
80. Astrup A, Caterson I, Zelissen P, Guy-Grand B, Carruba M, Levy B, et al. Topiramate: long-term
maintenance of weight loss induced by a low-calorie diet in obese subjects. Obes Res
2004;12(10):1658-69.
81. Wadden TA, Fujioka K, Toubro S, Gantz I, Erondu NE, Chen M, et al. A randomized trial of
lifestyle modification and taranabant for maintaining weight loss achieved with a low-calorie
diet. Obesity (Silver Spring) 2010;18(12):2301-10.
82. Anderson JW, Konz EC, Frederich RC, Wood CL. Long-term weight-loss maintenance: a meta-
analysis of US studies. Am J Clin Nutr 2001;74(5):579-84.
83. Saris WH. Very-low-calorie diets and sustained weight loss. Obes Res 2001;9 Suppl 4:295S-301S.
84. Maddrey WC, Schiff ER, Sorrel MF, Ovid. Schiff's diseases of the liver. Chapter 22. Gal stone Disease:
Pathogenesis and Treatment. 10th ed. Philadelphia: Lippincott Wil iams & Wilkins, 2007:p.640-63.
85. Schirmer BD, Winters KL, Edlich RF. Cholelithiasis and cholecystitis. Journal of long-term effects of
medical implants 2005;15(3):329-38.
86. Stinton LM, Myers RP, Shaffer EA. Epidemiology of gallstones. Gastroenterology clinics of North
America 2010;39(2):157-69, vi .
87. Erlinger S. Gal stones in obesity and weight loss. European journal of gastroenterology & hepatology
2000;12(12):1347-52.
88. Everhart JE. Contributions of obesity and weight loss to gallstone disease. Annals of internal medicine
1993;119(10):1029-35.
89. Festi D, Colecchia A, Larocca A, Villanova N, Mazzel a G, Petroni ML, et al. Review: low caloric
intake and gal -bladder motor function. Alimentary pharmacology & therapeutics 2000;14 Suppl
90. Broomfield PH, Chopra R, Sheinbaum RC, Bonorris GG, Silverman A, Schoenfield LJ, et al. Effects
of ursodeoxycholic acid and aspirin on the formation of lithogenic bile and gallstones during
loss of weight. The New England journal of medicine 1988;319(24):1567-72.
91. Kamrath RO, Plummer LJ, Sadur CN, Adler MA, Strader WJ, Young RL, et al. Cholelithiasis in
patients treated with a very-low-calorie diet. The American journal of clinical nutrition 1992;56(1
Suppl):255S-57S.
92. Liddle RA, Goldstein RB, Saxton J. Gal stone formation during weight-reduction dieting. Archives of
internal medicine 1989;149(8):1750-3.
93. Shiffman ML, Kaplan GD, Brinkman-Kaplan V, Vickers FF. Prophylaxis against gallstone formation
with ursodeoxycholic acid in patients participating in a very-low-calorie diet program. Annals of
internal medicine 1995;122(12):899-905.
94. Yang H, Petersen GM, Roth MP, Schoenfield LJ, Marks JW. Risk factors for gallstone formation
during rapid loss of weight. Digestive diseases and sciences 1992;37(6):912-8.
95. Festi D, Colecchia A, Orsini M, Sangermano A, Sottili S, Simoni P, et al. Gallbladder motility and
gallstone formation in obese patients fol owing very low calorie diets. Use it (fat) to lose it
(wel ). International journal of obesity and related metabolic disorders : journal of the International
Association for the Study of Obesity 1998;22(6):592-600.
96. Gebhard RL, Prigge WF, Ansel HJ, Schlasner L, Ketover SR, Sande D, et al. The role of gal bladder
emptying in gallstone formation during diet-induced rapid weight loss. Hepatology
1996;24(3):544-8.
97. Hoy MK, Heshka S, Al ison DB, Grasset E, Blank R, Abiri M, et al. Reduced risk of liver-function-
test abnormalities and new gallstone formation with weight loss on 3350-kJ (800-kcal) formula
diets. The American journal of clinical nutrition 1994;60(2):249-54.
98. Spirt BA, Graves LW, Weinstock R, Bartlett SJ, Wadden TA. Gal stone formation in obese women
treated by a low-calorie diet. International journal of obesity and related metabolic disorders :
journal of the International Association for the Study of Obesity 1995;19(8):593-5.
99. Young T, Palta M, Dempsey J, Skatrud J, Weber S, Badr S. The occurrence of sleep-disordered
breathing among middle-aged adults. N Engl J Med 1993;328(17):1230-5.
100. The-report-of-an-American-Academy-of-Sleep-Medicine-Task-Force. Sleep-related breathing
disorders in adults: recommendations for syndrome definition and measurement techniques in
clinical research. The Report of an American Academy of Sleep Medicine Task Force. Sleep
1999;22(5):667-89.
101. Punjabi NM. The epidemiology of adult obstructive sleep apnea. Proc Am Thorac Soc 2008;5(2):136-
102. Young T, Peppard PE, Gottlieb DJ. Epidemiology of obstructive sleep apnea: a population health
perspective. Am J Respir Crit Care Med 2002;165(9):1217-39.
103. Lindberg E, Gislason T. Epidemiology of sleep-related obstructive breathing. Sleep Med Rev
2000;4(5):411-33.
104. Schwartz AR, Patil SP, Laffan AM, Polotsky V, Schneider H, Smith PL. Obesity and obstructive
sleep apnea: pathogenic mechanisms and therapeutic approaches. Proc Am Thorac Soc
2008;5(2):185-92.
105. Newman AB, Foster G, Givelber R, Nieto FJ, Redline S, Young T. Progression and regression of
sleep-disordered breathing with changes in weight: the Sleep Heart Health Study. Arch Intern
Med 2005;165(20):2408-13.
106. Peppard PE, Young T, Palta M, Dempsey J, Skatrud J. Longitudinal study of moderate weight
change and sleep-disordered breathing. JAMA 2000;284(23):3015-21.
107. Tishler PV, Larkin EK, Schluchter MD, Redline S. Incidence of sleep-disordered breathing in an
urban adult population: the relative importance of risk factors in the development of sleep-
disordered breathing. JAMA : the journal of the American Medical Association 2003;289(17):2230-7.
108. Pil ar G, Shehadeh N. Abdominal fat and sleep apnea: the chicken or the egg? Diabetes Care
2008;31 Suppl 2:S303-9.
109. Banno K, Kryger MH. Sleep apnea: clinical investigations in humans. Sleep Med 2007;8(4):400-26.
110. Punjabi NM, Caffo BS, Goodwin JL, Gottlieb DJ, Newman AB, O'Connor GT, et al. Sleep-
disordered breathing and mortality: a prospective cohort study. PLoS Med 2009;6(8):e1000132.
111. Marshal NS, Wong KK, Liu PY, Cul en SR, Knuiman MW, Grunstein RR. Sleep apnea as an
independent risk factor for al -cause mortality: the Busselton Health Study. Sleep
2008;31(8):1079-85.
112. Young T, Finn L, Peppard PE, Szklo-Coxe M, Austin D, Nieto FJ, et al. Sleep disordered breathing
and mortality: eighteen-year fol ow-up of the Wisconsin sleep cohort. Sleep 2008;31(8):1071-8.
113. de Sousa AG, Cercato C, Mancini MC, Halpern A. Obesity and obstructive sleep apnea-hypopnea
syndrome. Obes Rev 2008;9(4):340-54.
114. Johansson K, Neovius M, Lagerros YT, Harlid R, Rossner S, Granath F, et al. Effect of a very low
energy diet on moderate and severe obstructive sleep apnoea in obese men: a randomised
control ed trial. BMJ 2009;339:b4609.
115. Tuomilehto HP, Seppa JM, Partinen MM, Peltonen M, Gyl ing H, Tuomilehto JO, et al. Lifestyle
intervention with weight reduction: first-line treatment in mild obstructive sleep apnea. Am J
Respir Crit Care Med 2009;179(4):320-7.
116. Foster GD, Borradaile KE, Sanders MH, Mil man R, Zammit G, Newman AB, et al. A randomized
study on the effect of weight loss on obstructive sleep apnea among obese patients with type 2
diabetes: the Sleep AHEAD study. Arch Intern Med 2009;169(17):1619-26.
117. Dempsey JA, Veasey SC, Morgan BJ, O'Donnel CP. Pathophysiology of sleep apnea. Physiol Rev
2010;90(1):47-112.
118. SBU-rapport. Obstructive sleep apnoea syndrome : a systematic literature review. Stockholm: SBU,
119. Shneerson J, Wright J. Lifestyle modification for obstructive sleep apnoea. Cochrane Database Syst
Rev 2001(1):CD002875.
120. Grunstein RR, Stenlof K, Hedner JA, Peltonen M, Karason K, Sjostrom L. Two year reduction in
sleep apnea symptoms and associated diabetes incidence after weight loss in severe obesity.
Sleep 2007;30(6):703-10.
121. Schwartz AR, Gold AR, Schubert N, Stryzak A, Wise RA, Permutt S, et al. Effect of weight loss on
upper airway col apsibility in obstructive sleep apnea. Am Rev Respir Dis 1991;144(3 Pt 1):494-8.
122. Smith PL, Gold AR, Meyers DA, Haponik EF, Bleecker ER. Weight loss in mildly to moderately
obese patients with obstructive sleep apnea. Annals of internal medicine 1985;103(6 ( Pt 1)):850-
123. Barnes M, Goldsworthy UR, Cary BA, Hil CJ. A diet and exercise program to improve clinical
outcomes in patients with obstructive sleep apnea--a feasibility study. Journal of clinical sleep
medicine : JCSM : official publication of the American Academy of Sleep Medicine 2009;5(5):409-15.
124. Dixon JB, Schachter LM, O'Brien PE. Polysomnography before and after weight loss in obese
patients with severe sleep apnea. Int J Obes (Lond) 2005;29(9):1048-54.
125. Fritscher LG, Canani S, Mottin CC, Fritscher CC, Berleze D, Chapman K, et al. Bariatric surgery
in the treatment of obstructive sleep apnea in morbidly obese patients. Respiration
2007;74(6):647-52.
126. Haines KL, Nelson LG, Gonzalez R, Torrella T, Martin T, Kandil A, et al. Objective evidence that
bariatric surgery improves obesity-related obstructive sleep apnea. Surgery 2007;141(3):354-8.
127. Hakala K, Maasilta P, Sovijarvi AR. Upright body position and weight loss improve respiratory
mechanics and daytime oxygenation in obese patients with obstructive sleep apnoea. Clin
Physiol 2000;20(1):50-5.
128. Hernandez TL, Bal ard RD, Weil KM, Shepard TY, Scherzinger AL, Stamm ER, et al. Effects of
maintained weight loss on sleep dynamics and neck morphology in severely obese adults.
Obesity (Silver Spring) 2009;17(1):84-91.
129. Kajaste S, Brander PE, Telakivi T, Partinen M, Mustajoki P. A cognitive-behavioral weight reduction
program in the treatment of obstructive sleep apnea syndrome with or without initial nasal
CPAP: a randomized study. Sleep Med 2004;5(2):125-31.
130. Kajaste S, Telakivi T, Mustajoki P, Pihl S, Partinen M. Effects of a cognitive-behavioural weight loss
programme on overweight obstructive sleep apnoea patients. Journal of sleep research
1994;3(4):245-49.
131. Kansanen M, Vanninen E, Tuunainen A, Pesonen P, Tuononen V, Hartikainen J, et al. The effect of
a very low-calorie diet-induced weight loss on the severity of obstructive sleep apnoea and
autonomic nervous function in obese patients with obstructive sleep apnoea syndrome. Clin
Physiol 1998;18(4):377-85.
132. Lettieri CJ, Eliasson AH, Greenburg DL. Persistence of obstructive sleep apnea after surgical
weight loss. Journal of clinical sleep medicine : JCSM : official publication of the American Academy of
Sleep Medicine 2008;4(4):333-8.
133. Lojander J, Mustajoki P, Ronka S, Mecklin P, Maasilta P. A nurse-managed weight reduction
programme for obstructive sleep apnoea syndrome. J Intern Med 1998;244(3):251-5.
134. Noseda A, Kempenaers C, Kerkhofs M, Houben JJ, Linkowski P. Sleep apnea after 1 year
domiciliary nasal-continuous positive airway pressure and attempted weight reduction.
Potential for weaning from continuous positive airway pressure. Chest 1996;109(1):138-43.
135. Pil ar G, Peled R, Lavie P. Recurrence of sleep apnea without concomitant weight increase 7.5
years after weight reduction surgery. Chest 1994;106(6):1702-4.
136. Suratt PM, McTier RF, Findley LJ, Pohl SL, Wilhoit SC. Effect of very-low-calorie diets with weight
loss on obstructive sleep apnea. Am J Clin Nutr 1992;56(1 Suppl):182S-84S.
137. Valencia-Flores M, Orea A, Herrera M, Santiago V, Rebol ar V, Castano VA, et al. Effect of
bariatric surgery on obstructive sleep apnea and hypopnea syndrome, electrocardiogram, and
pulmonary arterial pressure. Obes Surg 2004;14(6):755-62.
138. Nerfeldt P, Nilsson BY, Mayor L, Udden J, Friberg D. A two-year weight reduction program in
obese sleep apnea patients. Journal of clinical sleep medicine : JCSM : official publication of the
American Academy of Sleep Medicine 2010;6(5):479-86.
139. Johansson K, Hemmingsson E, Harlid R, Trol e Lagerros Y, Granath F, Rossner S, et al. Longer
term effects of very low energy diet on obstructive sleep apnoea in cohort derived from
randomised control ed trial: prospective observational fol ow-up study. BMJ 2011;342:d3017.
140. Tuomilehto H, Gylling H, Peltonen M, Martikainen T, Sahlman J, Kokkarinen J, et al. Sustained
improvement in mild obstructive sleep apnea after a diet- and physical activity-based lifestyle
intervention: postinterventional fol ow-up. Am J Clin Nutr 2010;92(4):688-96.
141. Tsai AG, Wadden TA. Surgery decreases long-term mortality, morbidity, and health care use in
morbidly obese patients. Annals of surgery 2005;242(2):290.
142. Moroshko I, Brennan L, O'Brien P. Predictors of dropout in weight loss interventions: a
systematic review of the literature. Obes Rev 2011;12(11):912-34.
143. Melin I, Karlstrom B, Lappalainen R, Berglund L, Mohsen R, Vessby B. A programme of behaviour
modification and nutrition counsel ing in the treatment of obesity: a randomised 2-y clinical
trial. Int J Obes Relat Metab Disord 2003;27(9):1127-35.
144. Melin I, Reynisdottir S, Berglund L, Zamfir M, Karlstrom B. Conservative treatment of obesity in
an academic obesity unit. Long-term outcome and drop-out. Eat Weight Disord 2006;11(1):22-
145. Melin I. Obesitas : Arbetsbok - för dig som vil gå ner i vikt. 2. uppl. ed. Lund: Studentlitteratur, 2001.
146. Melin I. Obesitas : handbok för praktisk klinisk behandling av övervikt, fetma och metabolt syndrom
baserad på kognitiv beteendemodifikation och konventionel behandling. 2. uppl. ed. Lund:
Studentlitteratur, 2001.
147. Harris JA, Benedict FG. A Biometric Study of Basal Metabolism in Man. Washington DC: The
Carnegie Institute 1912.
148. Livsmedelsverket. Svenska näringsrekommendationer - Rekommendationer om näring och fysisk aktivite.
4. uppl. ed. Uppsala: Livsmedelsverket, 2005.
149. Sul ivan M, Karlsson J, Taft C. SF-12 Health Survey: Swedish User Handbook. Gothenburg:
Sahlgrenska University Hospital, 1997.
150. Vickers AJ, Altman DG. Statistics notes: Analysing control ed trials with baseline and fol ow up
measurements. BMJ 2001;323(7321):1123-4.
151. Bland JM, Altman DG. Matching. BMJ 1994;309(6962):1128.
152. Borenstein M. Introduction to meta-analysis. 1st ed. U.K.: Chichester, West Sussex, 2009.
153. Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med
2002;21(11):1539-58.
154. Ware JH. Interpreting incomplete data in studies of diet and weight loss. The New England journal
of medicine 2003;348(21):2136-7.
155. Veasey SC, Guilleminault C, Strohl KP, Sanders MH, Bal ard RD, Magalang UJ. Medical therapy for
obstructive sleep apnea: a review by the Medical Therapy for Obstructive Sleep Apnea Task
Force of the Standards of Practice Committee of the American Academy of Sleep Medicine.
Sleep 2006;29(8):1036-44.
156. Jonas E, Marsk R, Rasmussen F, Freedman J. Incidence of postoperative gallstone disease after
antiobesity surgery: population-based study from Sweden. Surgery for obesity and related diseases
: official journal of the American Society for Bariatric Surgery 2010;6(1):54-8.
157. Torgerson JS, Lindroos AK, Naslund I, Peltonen M. Gal stones, gal bladder disease, and
pancreatitis: cross-sectional and 2-year data from the Swedish Obese Subjects (SOS) and SOS
reference studies. The American journal of gastroenterology 2003;98(5):1032-41.
158. Padwal R, Li SK, Lau DC. Long-term pharmacotherapy for obesity and overweight. Cochrane
Database Syst Rev 2003(4):CD004094.
159. Barton S. Which clinical studies provide the best evidence? The best RCT stil trumps the best
observational study. BMJ 2000;321(7256):255-6.
160. Levendowski DJ, Zack N, Rao S, Wong K, Gendreau M, Kranzler J, et al. Assessment of the test-
retest reliability of laboratory polysomnography. Sleep Breath 2009;13(2):163-7.
161. Bar A, Pillar G, Dvir I, Sheffy J, Schnall RP, Lavie P. Evaluation of a portable device based on
peripheral arterial tone for unattended home sleep studies. Chest 2003;123(3):695-703.
162. Zou D, Grote L, Peker Y, Lindblad U, Hedner J. Validation a portable monitoring device for sleep
apnea diagnosis in a population based cohort using synchronized home polysomnography. Sleep
2006;29(3):367-74.
163. Gripeteg L, Karlsson J, Torgerson J, Lindroos AK. Predictors of very-low-energy diet outcome in
obese women and men. Obesity facts 2010;3(3):159-65.
Source: http://www.itrim.de/globalassets/se/pdf/forskningsrapporter/thesis_kari_johansson.pdf
Catalogo unificato genitorialita' e famiglia
Rassegna bibliografica Genitorialità/Famiglia Genitorialità/Famiglia L'educazione (im)possibile Orientarsi in una società senza padri Vittorino Andreoli Rizzoli Editore, 2014 Numero pagine: 213 Educare oggi, sostiene Andreoli, vuol dire insegnare a vivere in un mondo vastissimo e così mutevole da diventare quasi misterioso. Come fare? Come si può e si deve immaginare l'educazione in una società camaleontica dove tutto si trasforma continuamente, compresi i sentimenti e legami umani (parte indispensabile di ogni processo di crescita)? Da questa domanda parte un grido d'allarme che coinvolge non solo la famiglia e la scuola ma l'intera società, giacché il fallimento educativo è un malessere profondo che riguarda tutti, genitori e no, insegnanti e no, e che può essere risolto solo con uno sforzo comune (in primis ritrovando un punto d'unione con tutte le figure chiamate in causa durante la crescita dei ragazzi e tra loro una costante comunicazione tesa ad evitare la moltiplicazione degli stili educativi). Gli adulti devono capire, sottolinea Andreoli, che i sentimenti e i legami, come anche la possibilità di una progettualità a lunga durata (vale a dire della percezione del futuro da parte degli adolescenti), devono essere prioritari in quanto veicoli di messaggi che servono a dare sicurezza ed aiutano a formare l'identità del ragazzo: l'internet e i social networks potranno anche offrire agli adolescenti stimoli ed emozioni maggiori, ma senza ricchezza e benefici dei legami affettivi "reali". Il saggio ha un forte carattere divulgativo e cerca di dare risposte esaurienti a varie problematiche adolescenziali mettendo al centro di tutto la famiglia e la sua funzione: non più una somma di Io separati, ma una piccola orchestra diretta dal "bisogno esistenziale dell'uomo e della convivenza tra uomini". Informazioni su autore: Andreoli è considerato uno dei maggiori psichiatri italiani; al grande pubblico è noto in quanto studioso dei meccanismi della mente umana e osservatore del disagio psicologico degli adolescenti e dei loro genitori (argomento al quale ha dedicato, nel corso della sua carriera professionale, numerosi saggi) Altri soggetti: paternità e maternità/aspetti socio-culturali; educazione familiare; ruolo del contesto scolastico; adolescenza; identità
Microsoft word - ul lafayette h1n1 prep _2_.docx
UL Lafayette GENERAL PANDEMIC GUIDE Seasonal (common) Flu • Caused by: Human influenza virus • Transmitted: From person to person • Immunity: o Most people have some immunity o Vaccine is available Pandemic flu would describe a new human virus that: • Is easily spread throughout the world