Humanbehaviors.free.fr
164 • The Journal of Neuroscience, January 2, 2013 • 33(1):164 –174Neuroestrogens Rapidly Regulate Sexual Motivation But Not
Performance
Aurore L. Seredynski,1 Jacques Balthazart,1 Virginie J. Christophe,1 Gregory F. Ball,2 and Charlotte A. Cornil1
1Groupe Interdisciplinaire de Génoprotéomique Appliquée (GIGA) Neurosciences, Research Group in Behavioral Neuroendocrinology, University of Lie ge, 4000
Lie ge, Belgium, and 2Department of Psychological and Brain Sciences, Johns Hopkins University, Krieger School of Arts and Sciences, Baltimore, Maryland 21218
Estrogens exert pleiotropic effects on reproductive traits, which include differentiation and activation of reproductive behaviors and the
control of the secretion of gonadotropins. Estrogens also profoundly affect non-reproductive traits, such as cognition and neuroprotec-
tion. These effects are usually attributed to nuclear receptor binding and subsequent regulation of target gene transcription. Estrogens
also affect neuronal activity and cell-signaling pathways via faster, membrane-initiated events. How these two types of actions that
operate in distinct timescales interact in the control of complex behavioral responses is poorly understood. Here, we show that the central
administration of estradiol rapidly increases the expression of sexual motivation, as assessed by several measures of sexual motivation
produced in response to the visual presentation of a female but not sexual performance in male Japanese quail. This effect is mimicked by
membrane-impermeable analogs of estradiol, indicating that it is initiated at the cell membrane. Conversely, blocking the action of
estrogens or their synthesis by a single intracerebroventricular injection of estrogen receptor antagonists or aromatase inhibitors,
respectively, decreases sexual motivation within minutes without affecting performance. The same steroid has thus evolved complemen-
tary mechanisms to regulate different behavioral components (motivation vs performance) in distinct temporal domains (long- vs
short-term) so that diverse reproductive activities can be properly coordinated to improve reproductive fitness. Given the pleiotropic
effects exerted by estrogens, other responses controlled by these steroids might also depend on a slow genomic regulation of neuronal
plasticity underlying behavioral activation and an acute control of motivation to engage in behavior.
Although it was known that estrogen synthesis by testosterone Estrogens affect multiple reproductive endpoints, such as the (T) aromatization occurs in various tissues, including the brain development of secondary sexual characteristics, gonadotro- the recent demonstra- pin secretion, or activation of reproductive behaviors, but also tion that aromatase activity and brain estrogen concentrations non-reproductive traits, such as cognition. These effects typ- are regulated within minutes in vitro and in vivo by neuronal ically are associated with long-term changes in physiological state (e.g., a non-reproductive vs a reproductive state charac- suggests the exis- teristic of seasonally breeding species). They usually require a tence of rapidly changing local estrogen concentrations that relatively long (i.e., hours to days) exposure to gonadally de- could affect brain physiology in a shorter timeframe. In other rived estrogens and are mediated by nuclear actions of estro- words, in addition to seasonal changes in physiology resulting gens that modulate transcription through binding to their from variations in circulating concentrations of estrogens or its cognate nuclear receptors precursor T, local estrogen synthesis appears to be acutely regu- This original view of estrogen action has recently lated in the brain and could in turn affect neural activity in the undergone a radical transformation.
short term. This is a potentially exciting prospect because, duringthe reproductive season, many behavioral or physiological eventsare regulated on a much shorter timescale. For example, sexual Received May 27, 2012; revised Oct. 31, 2012; accepted Nov. 4, 2012.
behavior is not displayed continuously but rather is modulated Author contributions: A.L.S., J.B., G.F.B., and C.A.C. designed research; A.L.S. and V.J.C. performed research; J.B.
on a very short timescale. It was always assumed that such short- and C.A.C. contributed unpublished reagents/analytic tools; A.L.S., V.J.C., and C.A.C. analyzed data; A.L.S., J.B.,G.F.B., and C.A.C. wrote the paper.
term changes in behavior were regulated by neurotransmitter This research was supported by National Institute of Mental Health Grant MH50388 (J.B. and G.F.B.), the Belgian systems. Could steroids be responsible for both long-term and Basic Research Collective Grant 2.4537.09, and the University of Lie ge Special Funds 2009 (J.B.). C.A.C. is National short-term variations in behavioral production? Fund for Scientific Research research associate. We thank Catherine de Bournonville for her help with the statistical In vitro studies established that estrogens rapidly activate analysis. We are grateful to Dr. Margaret M. McCarthy and Dr. J. Martin Wild for their comments on previous versionsof this manuscript.
(within seconds to minutes) numerous intracellular signaling The authors declare no competing financial interests.
pathways, which in turn regulate neuronal activity Correspondence should be addressed to Charlotte A. Cornil, University of Lie ge, Groupe Interdisciplinaire These cellular effects are often transient and initiated by de Génoprotéomique Appliquée (GIGA) Neurosciences, Research Group in Behavioral Neuroendocrinology, 1 either nuclear receptors associated with the cell membrane avenue de l'Hopital (B36), 4000 Lie ge, Belgium. E-mail: [email protected].
or novel membrane estrogen Copyright 2013 the authors 0270-6474/13/330164-11$15.00/0 receptors Although some Seredynski et al. • Acute Effects of Neuroestrogens on Behavior J. Neurosci., January 2, 2013 • 33(1):164 –174 • 165
studies have identified rapid effects of estrogens on behavioral for 1 min before being removed to allow diffusion of the solution into the CSF and avoid its leakage outside of the brain. The internal cannula was including effects then slowly lifted and replaced by the dummy cannula.
on male copulatory behavior (for details, see The size of the cloacal gland, an androgen-dependent structure, was such evidence remains scarce. Moreover, evidence concerning periodically measured with calipers to confirm the effectiveness ofchronic T treatment of the birds because it was demonstrated previously male sexual behavior is often poorly reproducible that this size is a highly accurate and sensitive measure of peripheral T In particular, whether brain- concentrations and that variation in the size of this gland derived estrogens also affect male sexual behavior by non- integrates changes in circulating T over a long period of time genomic mechanisms and why these two types of signaling would Animals were also regularly weighed to have evolved to mediate estrogen action on the same response is confirm the absence of adverse effect of treatments on their general not known. The present study addresses the first of these ques- health condition.
tions in male Japanese quail, a well-established model for thestudy of the role of brain-derived estrogens (neuroestrogens) in male sexual behavior Our findings Previous studies have shown that a single systemic administration of indicate that estrogens have evolved complementary mecha- estradiol (E ) or of an aromatase inhibitor, mimicking an increased or nisms to regulate different behavioral components (motivation decreased bioavailability of estrogens, respectively, acutely facilitates or vs performance) in distinct temporal domains.
reduces male sexual behavior in birds as well as rodents However, thesestudies did not provide any information regarding the origin of the es- Materials and Methods
trogens that modulate behavior (brain vs periphery) and suffered from high variability and/or poor reproducibility. These issues might partially Forty-three male Japanese quail (Coturnix japonica) belonging to three be circumvented by administering treatments directly in the brain rather independent groups served as subjects in these experiments. Birds were than in the periphery. In addition, the detection of these acute effects of obtained from the breeding colony established in our laboratory (groups estrogens on behavior may critically depend on the endocrine status of 1 and 2) or a local breeder (group 3). In male quail, reproduction is the experimental animals that may constrain the potential interactions tightly controlled by variations in photoperiod that regulate both go- between genomic and nongenomic actions of estrogens (see Discussion).
nadal growth and gonadal regression To In the present study, we thus decided to use two separate but comple- fully stimulate their hypothalamic–pituitary– gonadal axis, birds were mentary approaches during 17 separate experiments performed on three exposed throughout their life to a photoperiod simulating long summer independent groups of birds to assess the acute effects of estrogens on days (16 h light/8 h dark), although these animals were castrated (CX) male sexual behavior. Both approaches involved the administration of and chronically supplemented with exogenous T (see below). They had various steroidal and nonsteroidal drugs directly into the brain via a food and water available ad libitum. All experimental procedures were in cannula implanted in the third ventricle.
agreement with the Belgian laws on the "Protection and Welfare of Ani- The first approach was based on our previous observation that the mals" and on the "Protection of Experimental Animals" and were ap- effects of acute estrogens cannot be detected in castrates or males exposed proved by the Ethics Committee for the Use of Animals at the University to physiological concentrations of estrogens (C.A.C. and J.B., unpub- lished observations) and were not observed consistently in males exposed All birds were castrated as described previously to low concentrations of T [and its androgenic and estrogenic metabolites; at the age of 3 weeks when the testes are still small and quiescent before Therefore, we decided in this first approach (experi- puberty. This manipulation has no effect on the sexual differentiation of ments 1–3 on birds from group 1) to mimic an increase in E bioavailability the individuals because the window framing the critical period for sexual by acutely injecting E to CX males chronically treated with T but also chron- differentiation has been long closed by this time ically deprived of estrogens through twice daily injections of the potent The birds then remained housed in groups until the age of 6 weeks, when aromatase inhibitor Vorozole (6-[(4-chlorophenyl)(1 H-1,2,4-triazol-1- they were moved to individual cages. At the age of 8 weeks, all birds were yl)methyl]-1-methyl-1 H-benzotriazole) (VOR). These males are thus ex- implanted in the third ventricle with a chronic 22 gauge injection guide posed to the full range of purely androgenic effects but are not subjected to cannula containing a 28 gauge dummy insert (C313G and C313I; Plastics any estrogenic action (genomic or nongenomic). It was hypothesized that, if One). Coordinates of the cannula tip were 1.80 mm anterior, 2.80 mm nongenomic actions of estrogens are involved in the control of male sexual dorsal, and 0.00 mm lateral to the zero reference point (center of the behavior, E injections should acutely restore parts of this behavior unless interaural axis) using an angular approach (10° away from the vertical) to some degree of genomic priming by estrogens (e.g., E -induced induction of avoid the sagittal sinus present in medial position at the surface of the transcription of a membrane estrogen receptor) is required for nongenomic brain (see detail of methods in the study by The actions to take place. Although one could expect that the long-term blockade location of the cannula in the ventricle was confirmed at implantation of aromatase would result in an elevation of T concentrations, a very small and before any injection by the observation of a drop of CSF flowing out proportion of aromatizable androgens only (far less than 1%) is actually of the tip of the cannula when the dummy insert was removed converted locally into estrogens in avian and mammalian species All birds were then implanted As a consequence, the subcutaneously in the neck region with one 20-mm-long SILASTIC tube effects of chronic VOR treatment are unlikely the result from an elevation in (1.57 mm inner diameter, 2.41 mm outer diameter, Silclear Tubing; androgens, but they rather reflect the lack of a genomic action of estrogens.
Degania Silicone) filled with crystalline T (Sigma) to mimic the increase In contrast, the second approach (experiments 4 –17 on birds from in gonadal secretion occurring approximately at this age and fully acti- groups 2 and 3) was designed to mimic an acute reduction of estrogen vate male-typical behaviors bioavailability through the acute blockade of estrogen synthesis (by aro- Intracerebroventricular injections and behavioral tests were started 2 matase inhibitors) or action (by estrogen receptor antagonists) in CX weeks after the beginning of T treatment. Intracerebroventricular injec- males chronically treated with exogenous T such that they displayed the tions in the third ventricle were performed with a 5 l Hamilton syringe full suite of male-typical behaviors. If an effect of acute blockade of connected to a microinfusion pump (model KDS-220; KD Scientific).
aromatization was detected, it was then planned to assess the reversibility The dummy cannula was replaced by a 28 gauge internal cannula of this effect by injecting various estrogenic compounds to determine the (C313C; Plastics One) attached to the syringe by a cannula connector.
specificity of the response.
The liquid (1 or 2 l depending on the dose and drug) was infused at the These two approaches are complementary in that (1) they analyze the rate of 0.5 l/min, resulting in injection durations of 2 and 4 min for the mechanisms leading to the activation as well as the suppression of the injection of 1 or 2 l, respectively. The internal cannula was left in place same behavioral response and (2) they should provide insights into 166 • J. Neurosci., January 2, 2013 • 33(1):164 –174
Seredynski et al. • Acute Effects of Neuroestrogens on Behavior the interplay between genomic and non- genomic actions of estrogens in the control of the same behavioral output.
Three groups of animals were used for the Day 1 Day 2
three different series of experiments. Regard-less of the group considered, all animals wererepeatedly tested with all drugs in a within- subject design. Practically, the animals werefirst tested (see specific experiments design) toensure that they were in the appropriate condi- tions (preliminary phase). When ready to enter the acute phase of the experiment (see details ofspecific experiments), birds were assigned todifferent subgroups that did not differ with re- spect to their average behavioral frequencies displayed during the preliminary phase nor to morphological features (cloacal gland size and Day 1 Day 2 Day 3
body mass). These subgroups were repeatedlytested on different days with different drugs (one drug vs its vehicle or two drugs vs theirvehicle; for details of design, see buteach subgroup received these drugs in a differ- ent order. This procedure allowed us to controlfor any long-lasting effect of the drugs attribut-able to the activation of genomic actions or the slow clearance of the drug in the birds. In ad-dition, to ensure that the treatments did notexert long-lasting effects on the behavior, allbirds of groups 2 and 3 were pretested after a Figure 1.
Experimental design used when comparing the effect of one (A) or two (B) experimental (Exp.) treatment(s) to their
vehicle injection before each series of experi- vehicle (Veh). Before the experiment, birds were divided into two or three subgroups (SG1–SG2 or SG1–SG3) depending on the mental treatments (pretest), as well as after number of conditions compared. Each experiment consisted in four or five tests: one pretest (Pre), two (A) or three (B) experimental
them (posttest). The vehicle injection was al- tests, and a posttest (Post). In the pretest, all animals regardless of the their subgroup were tested for a given behavioral response ways given following the same timing as the after an acute intracerebroventricular injection of vehicle. Each subgroup was then tested on two (A) or three (B) different days for
timing of the experimental drug(s) tested. The the two (A) or three (B) different treatments, but each subgroup received these treatments in different order such that, for
comparison of pretest and posttest allowed us example, in A, one half the animals would receive the experimental treatment on day 1 and the vehicle on day 2, whereas the other
to evaluate the stability of the behavioral re- half received the vehicle first and experimental treatment second. In the posttest, all birds were tested again after an injection of sponses despite multiple experimental treat- vehicle. In these experiments, all tests were given either 2 d (group 1) or 3 d apart (groups 2 and 3).
ments. The combination of the pretest, themultiple tests comparing one or two compounds with the vehicle (con- this test, the opaque panel was raised, allowing visual access to the female trol), and the posttest constitutes an experiment The interval although the male could still not physically interact with her. The num- between individual tests varied depending on the experimental design in ber of RCSM was recorded for 2.5 min before (phase 1) and during which the groups were tested. Each group of birds participated in several (phase 2) the view of the female. Several lines of evidence indicate that experiments testing the effects of different drugs, administered at differ- this measure is indicative of the male's motivation to approach its mate.
ent doses and timings, on different behavioral measures (see detail in the Indeed, previous studies showed that the RCSM frequency (1) rapidly increases in sexually active males provided with the view of a female but These experiments investigated rapid controls by estrogens of both the not when provided with a view of a male appetitive (variable behaviors that serve to bring individuals in contact (2) is readily displayed by naive males with their sexual partner, often used as a measure of sexual motivation, without previous sexual learning but can also be conditioned and elicited e.g., courtship) and consummatory (highly stereotyped behaviors allow- by an arbitrary stimulus previously associated with the presentation of a ing sperm transfer and leading to the reduction of sexual motivation, i.e., sexual stimulus, such as visual access to a female sexual satiety) aspects of male sexual behavior. Both components are (3) decreases after castra- activated by prolonged genomic effects of estrogens tion but is restored after supplementation with exogenous T but whether they are also regulated by (4) is reduced after copulation (M. Schmit, C. de Bournon- estrogens acting in a rapid mode was unclear when we started these ville, and C. A. Cornil, unpublished observations), and (5) is inhibited by lesions of the preoptic area, a brain region known to control male sexualbehavior Together, these observations strongly suggest that these cloacal contractions constitute a preparatory or appet- Appetitive sexual behavior itive response in anticipation of copulation that reflects the propensity of Two measures were used to assess appetitive sexual behavior (ASB): the male to engage in copulation (i.e., a measure of his underlying sexual the frequency of the rhythmic cloacal sphincter movements (RCSM) and the learned social proximity response (LSPR).
The LSPR is a form of associative learning in which males learn to The frequency of RCSM was quantified by placing the experimental stand in front of a narrow window in which they had previously been able male in one side of a glass aquarium (40 ⫻ 20 ⫻ 25 cm) located on a to see a sexually receptive female Animals used raised platform with a mirror placed underneath at a 45° angle that here were allowed to acquire and express this response in conditions provided an unobstructed view of the cloacal area similar to those described previously Briefly, the During the first phase of the test, a conditioning and tests took place in four two-compartment chambers.
stimulus female was placed on the other side of the aquarium separated Each chamber consisted of a large compartment (90 ⫻ 90 ⫻ 50 cm) from the male by a glass partition and a vertically sliding opaque panel where the male is placed at the beginning of each test and a smaller that prevented the animals from seeing each other. In the second phase of adjacent compartment (20 ⫻ 60 ⫻ 24 cm) housing the stimulus female.
Seredynski et al. • Acute Effects of Neuroestrogens on Behavior
J. Neurosci., January 2, 2013 • 33(1):164 –174 • 167
The two compartments are separated by a vertically sliding door that can
Table 1. Details of experiments 1–3 conducted on the first group of males
be remotely controlled by strings and pulleys. A narrow vertical slit (1cm
wide ⫻ 15 cm high) is located in the middle of this door and serves as a"window" that provides the male with a limited visual access to the
female. A square area (30 ⫻ 30 cm) located on the floor of the main
compartment in front of this door represents the test zone for the bird's
position. The male present in the main compartment can only see the
The experiments numbers (1–3) correspond to the order in which they were performed.
female located in the side compartment if he stands in front of the win-
aE –BSA was administered at a dose equivalent to 50 g of free estradiol following information provided by the
dow in the test area. A male learns to stand continuously in front of the
window providing visual access to the female after he has had a chance tocopulate with her in the experimental setup. Only one copulation isnecessary to establish this response.
Each behavioral test lasted a total of 10 min. The male was first placed
in the main compartment, and the stimulus female was introduced in theadjacent smaller compartment. The amount of time that the male spentin the square test area in front of the window (Square) as well as the timethat he actually spent looking through the window (Look) was recordedfor 5 min. Looking behavior was defined as a stereotyped positioning ofthe male's head such that he could focus on the female through thenarrow slit in the door. These data provided a measure of the appetitivebehavior. Then, the door separating the two compartments was raised,allowing the two birds to physically interact for another 5 min, duringwhich the frequency and latency of first occurrence of copulatory behav-iors were directly recorded (see below). The animals were then moved
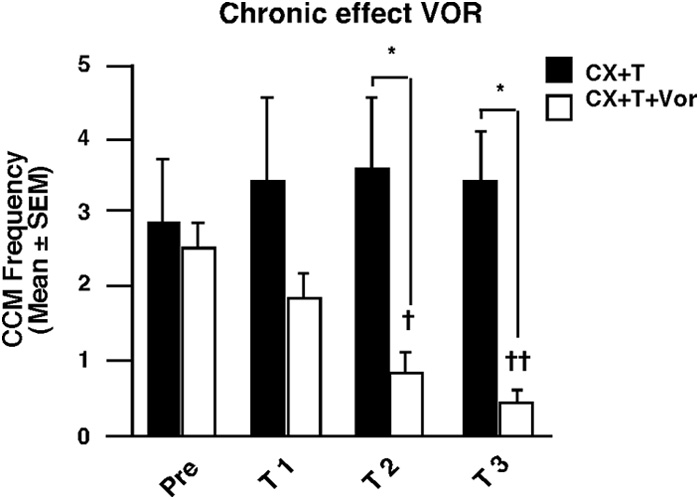
Figure 2.
Chronic treatment with the aromatase inhibitor VOR almost completely eliminates
back to their home cage until the next day.
CSB (group 1, preliminary phase). After the last pretest (Pre), CX males chronically treated with
In the present experiment, eight acquisition tests were performed on 8
T were injected twice daily with VOR (1 mg/kg, i.m.; white bars, CX ⫹ T ⫹ VOR, n ⫽ 12) or its
successive days that allowed all males to acquire a stable conditioned
vehicle (PG; controls, black bars, CX ⫹ T, n ⫽ 4). Birds were then repeatedly tested every third
response. Birds were then tested in extinction (female not released during
day for the expression of CSB. CCM frequencies gradually decreased to almost zero within 9 d.
the second period of 5 min) in two additional tests to ensure that, as
Data were analyzed by two-way ANOVA with treatment as independent factor (F
previously described, the response remains stable even in the absence of
p ⫽ 0.308) and successive tests as repeated factor (F
⫽ 17.532, p ⬍ 0.001; interaction,
reinforcement The
⫽ 3.758, p ⫽ 0.016). *p ⬍ 0.05 versus controls (CX ⫹ T); †p ⬍ 0.05 and ††p ⬍ 0.01
effect of different endocrine treatments was then tested as detailed in the
versus Pre same treatment, by Tukey's post hoc test.
specific section devoted to group 3. Importantly, the acquisition of thissocial proximity response occurs (1) only in gonadally intact males orcastrates chronically treated with T (2)
n ⫽ 4) for the entire duration of the experiment (25 d). To monitor the
only if males have been able to copulate in this experimental setup
behavioral decline induced by this prolonged deprivation in estrogens
and (3) in response to female but not male
copulatory behavior was first evaluated before the
stimuli Additionally, (4) the learned response is
first VOR injection. Starting on the second day after the beginning of the
markedly reduced after lesions of the preoptic area
twice daily injection regimen, birds were then subjected to three addi-
Together, these observations indicate that this learned response
tional tests for copulatory behavior until a significant behavioral decline
constitutes another good measure of ASB and reflects the propensity of
was detected In a last test, all birds then received a vehicle intra-
the male to engage in copulation (i.e., a measure of his underlying sexual
cerebroventricular injection and were tested in sequence for RCSM and
copulatory behavior in the aquarium in which all experimental tests wereperformed subsequently. This pretest confirmed the behavioral inhibi-
Consummatory sexual behavior
tion affecting both RCSM and CCM observed previously. All behavioral
Consummatory sexual behavior (CSB) was assessed after presentation of
tests took place 2 h after the morning injection and were given 3 d apart.
a sexually mature female with which the male could freely interact during
Experimental phase. VOR-treated birds were assigned to two experi-
5 min in three different test arenas: a small (60 ⫻ 40 ⫻ 50 cm; used during
mental subgroups (n ⫽ 6 each) matched based on the mean behavioral
all preliminary phases and for experimental tests in all groups) or large
frequencies displayed during the last preliminary test. These birds were
arena (90 ⫻ 90 ⫻ 50 cm; used for experimental tests in groups 2 and 3
involved in three experiments comparing the effect of the
only) or the small aquarium used to quantify RCSM after removal of the
control injection of the vehicle (PG) with either 50 g of E (in 1 l;
glass partition between the two compartments immediately after the
experiment 1), 100 g of E (in 2 l; experiment 2), or with its
RCSM test (during the experimental phase in group 1). The frequency of
membrane-impermeant analog E coupled with bovine serum albumin
behavioral patterns that are part of the copulatory sequence, including
(E –BSA; for more discussion on this compound, see below, Drugs; 50
neck grabs, mount attempts, mounts, and cloacal contact movements
g of E equivalent in 2 l; experiment 3). Birds were tested every other
(CCM) (for a detailed description of these behaviors, see
day for 15 min after the intracerebroventricular injection of the experi-
was recorded by an observer blind to the
mental drug or the vehicle. As described in , treatments were
treatments. Data relative to these four behavioral measures have been
administered in a different order to the two subgroups, such that all birds
analyzed and yielded the same statistical results and conclusions. As a
served as their own control but half of them were first treated with the
consequence, to avoid redundancy, only CCM results are shown here.
vehicle whereas the other half received the experimental injection.
Within the same experiment, animals were tested sequentially for ASB
Specific experimental procedures
and CSB in the glass aquarium used for ASB quantification. As a conse-
Effects of acute estrogen treatment in males chronically deprived of
quence, the test lasted a total of 10 min: 2.5 min for RCSM in the absence
estrogens (first group of birds)
of the female, 2.5 min for RCSM in the presence of the female, and 5 min
Preliminary phase. Two weeks after T implantation, 16 males were pre-
for CCM. The control birds that were not treated with VOR (i.e., CX ⫹ T
tested in small test chambers until they all displayed the full range of
birds) were tested in parallel after a control intracerebroventricular in-
copulatory behaviors. They were then injected twice daily in the pectoral
jection of PG. These animals served as a reference point for fully active
muscles with the potent aromatase inhibitor VOR (1 mg/kg; n ⫽ 12;
birds black histogram bars) but were not included in the
or its vehicle propylene glycol (PG)/saline (4:1;
statistical analysis of the acute experiments because they were not tested
168 • J. Neurosci., January 2, 2013 • 33(1):164 –174
Seredynski et al. • Acute Effects of Neuroestrogens on Behavior
Table 2. Details of experiments 4 –13 conducted on the second group of males
VOR and VOR ⫹ E
VOR and VOR ⫹ E – biotin
The experiment numbers follow the order in which the results are described, but the actual order in which the experiments were performed is provided in the second column. When E was administered along with VOR, the dose and timing
of injection of both compounds are separated by the slash, with VOR being listed first.
Table 3. Details of experiments 14 –17 conducted on the third group of males
VOR versus VOR ⫹ E
VOR versus VOR ⫹ E
VOR versus VOR ⫹ E
VOR versus VOR ⫹ E
The experiment numbers follow the order in which the results are described, but the actual order in which the experiments were performed is provided in the second column. Because VOR and E were administered with different timings,
the timing and doses of injections are both presented with VOR preceding E . LSPR, learned social proximity response.
with comparable experimental treatments. One bird lost its cannula be-
and had thus to be excluded from the experiments, resulting in a
tween the E and E –BSA tests and thus had to be excluded from exper-
decrease in degrees of freedom in the ANOVAs.
Effects of blockade of local estrogen action or synthesis in sexually
Effect of changes in brain-derived estrogens on the learned social
active males (second group of birds)
proximity response in sexually active males (third group of birds)
Preliminary phase. Two weeks after T implantation, birds (n ⫽ 15) were
Preliminary phase. Two weeks after T implantation, birds (n ⫽ 18) were
offered several opportunities to copulate with a female to allow them to
offered several opportunities to copulate with a female to acquire the full
express the full repertoire of copulatory behavior and were tested once
repertoire of copulatory behavior and tested once for RCSM to habituate
for RCSM to habituate them to the test arena.
to the test arena. As described above (see Behavioral tests), they received
Experimental phase. Based on the behavioral frequencies displayed
eight acquisition tests given on 8 consecutive days until they had devel-
during the preliminary phase, birds were assigned to one of three exper-
oped a stable LSPR.
imental subgroups (n ⫽ 5 each) matched based on the mean RCSM and
Experimental phase. The effects of the experimental treatments (VOR
CCM frequency produced during the preliminary phase. A total of 10 exper-
vs VOR ⫹ E2 vs vehicle) were tested during the extinction phase of the
iments were performed, each consisting of three repeated experimental tests
learned social proximity response (that is when the female is no longer
(one vehicle vs two drugs) surrounded by two "baseline" tests (one pretest
released from her cage in the second step of this test) to prevent any
and one posttest) to assess the effects of the blockade of estrogen synthesis or
feedback from the experimentally induced decrease in copulatory behav-
action on the expression of ASB measured by RCSM and on CSB investi-
ior on the learned response (experiment 14) On the day after
gated in small or large arenas. The experiments compared the following
the last acquisition test, all birds received a first test in extinction. Two
drugs with the vehicle: two estrogen receptor antagonists [ICI 182,780
days later, they all received a vehicle intracerebroventricular injection 30
min before a second test, which constituted the reference test for the basal
triene-3,17-diol) (ICI), 100 g; and tamoxifen (TMX), 100 g]; two aroma-
response (pretest). Birds were then assigned to one of three experimental
tase inhibitors [VOR, 50 g; and 1,4,6-androstatrien-3,17-dione (ATD), 50
subgroups (n ⫽ 6 each) matched based on the time spent in the square
g]; and VOR (50 g) with or without E (50 g) or E –biotin (50 g of E
and time spent looking at the female in the second extinction test of the
equivalent; a membrane-impermeant E analog; for more discussion on this
LSPR test. Then, three subgroups were repeatedly tested with the three
compound, see below, Drugs). The effect of these drugs was tested after
experimental treatments administered intracerebroventricularly, each
latencies of 15 or 30 min depending on the experiment. The details of
test given 3 d apart. Three days after the last experimental test, all sub-
these experiments and the order in which they were actually conducted
groups were tested again after a vehicle intracerebroventricular injection
are represented in As illustrated in , each experiment
serving as the posttest. A total of six tests were thus performed during the
started with a pretest during which all birds were tested after a control
extinction phase; the experimental phase was conducted on the last five
intracerebroventricular injection of PG. Then, the three subgroups
(one pretest, three experimental tests, and one posttest). Two birds in one
were submitted to three independent tests assessing the effect of three
subgroup lost their cannula during this experiment and were thus re-
treatments administered in different orders on ASB or CSB measured
moved from the statistical analysis.
15 or 30 min after injection. All birds were thus used as their own
To replicate some of the data obtained with group 2, nine animals were
control, with the different subgroups receiving the same exact treat-
selected randomly among this group of birds and were reassigned to
ments but in different orders to avoid any possible effects in final
three different subgroups (n ⫽ 3) based on their mean CCM and RCSM
results of long-term consequences of the acute treatments. Each series
frequencies. They were then tested for the effects of VOR, VOR ⫹ E , or
of three repeated tests was followed by a posttest after another control
their vehicle (PG) on copulatory behavior measured in the small (exper-
intracerebroventricular injection. All tests were conducted 3 d apart
iment 15) and large (experiment 16) test arena and on RCSM frequencies
throughout the entire experiment. All compounds tested were diluted
(experiment 17; Table 3). As was done previously, these experiments
in 1 l of PG with the exception of the highest dose of E that was
consisted of three experimental tests given 3 d apart surrounded by one
diluted in 2 l and administered intracerebroventricularly. Note that
pretest and one posttest given 3 d before and after the first and last
three birds (one in each subgroup) lost their cannula during the study
experimental test, respectively (see and details of group 2).
Seredynski et al. • Acute Effects of Neuroestrogens on Behavior
J. Neurosci., January 2, 2013 • 33(1):164 –174 • 169
RCSM frequencies did not reach the samelevel as in control birds that were notchronically treated with VOR. A higherdose of E2 (100 g) increased RCSM fre-quency to the same extent (F
16.264, p ⫽ 0.002; vehicle, 26.5 ⫾ 8.7; E2,53.7 ⫾ 9.7; experiment 2). In contrast, E2(50 or 100 g) had little or no effect onCCM frequency (50 g: , F
2.500, p ⫽ 0.144, experiment 1; 100 g: nobehavior was displayed by any subject re-gardless of the treatment; experiment 2).
To determine whether this effect of E2 was
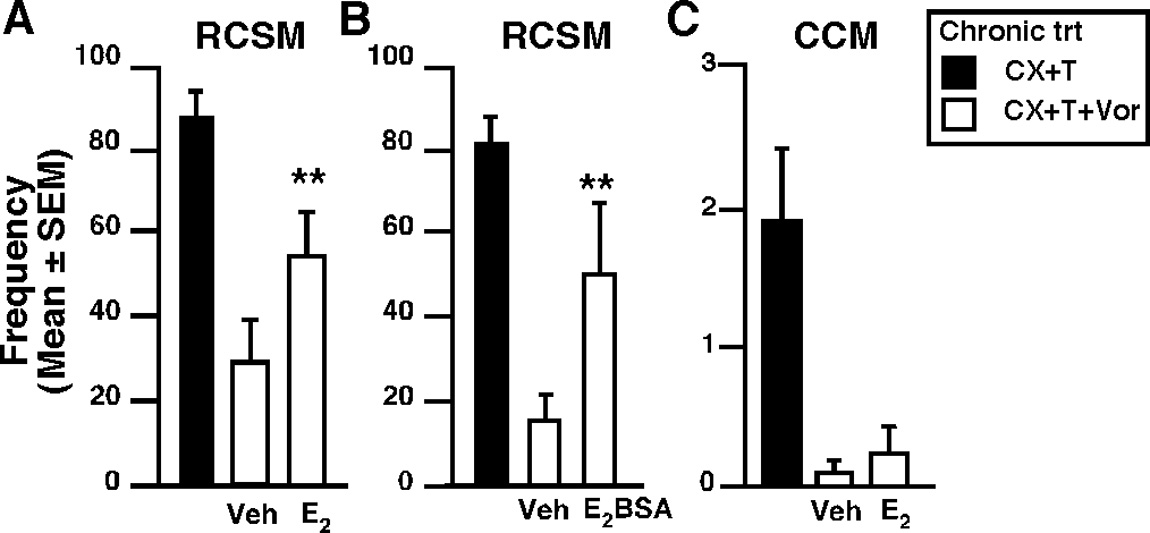
Figure 3.
E rapidly facilitates appetitive (ASB) but not consummatory sexual behavior (CSB) in males chronically deprived
mediated by its action at the membrane or
males of estrogens by its action at the membrane level (group 1, experimental phase). CX males were chronically treated with T and
inside the cells, we tested the effect of E2–
the aromatase inhibitor VOR (CX ⫹ T ⫹ VOR, white bars) or with its vehicle (CX ⫹ T; black bars, n ⫽ 4). The estrogen-deprived
BSA, which does not cross the cell mem-
males (CX ⫹ T ⫹ VOR) were then acutely injected with E (50 g, n ⫽ 12, A, C), the membrane-impermeant analog E –BSA (50
analog mirrored the effect of the free es-
respectively. Data from CX ⫹ T males (black bars) are shown as reference of fully stimulated behavior but were not integrated inthe final analyses. **p
trogen on RCSM frequencies ;
⬍ 0.01 versus vehicle by Fisher's LSD post hoc test after identification of a significant treatment effect
(repeated-measure) by two-way ANOVA.
12.41, p ⫽ 0.006; experiment 3),
indicating that this effect is initiated at thecell membrane. In contrast, no copulatory
behavior was displayed by any subject regardless of the treatment,
VOR was graciously provided by Dr. R. DeCoster (Janssen Research
suggesting the lack of a membrane-initiated effect on copulatory
Foundation, Beerse, Belgium). PG, 17-E , E –BSA (membrane-
impermeant analog of E ), ICI (Fulvestran), TMX, and T were
obtained from Sigma. ATD and E – biotin (another membrane-
No effect of the order of treatments (F ⱕ 0.659, p ⱖ 0.437) or
impermeant analog of E ) were obtained from Steraloids.
interaction between the treatments and their order (F ⱕ 2.979,
Two membrane-impermeant E analogs were used here. E –BSA is
p ⱖ 0.118) was detected with the exception of an interaction that
most commonly used in the literature, but there have been doubts re-
was detected for E
2 at 50 g ; F(1,10)
5.98, p ⫽ 0.034).
garding its stability and the purity of the commercially available product
However, this interaction is associated with a more pronounced
E – biotin was thus also used based on its recent use
effect of E2 in the subgroup that received E2 in the first test com-
for a similar purpose
pared with the second test, suggesting that it cannot be attributed
and the demonstration of a somewhat higher stability
to a long-term treatment effect. Overall, this absence of treatment
effects and interaction between treatments and orders suggeststhat there is no carryover effect indicative of long-term effects of
In each of the 17 separate experiments, results obtained in pretests and
Together, these data clearly indicate that a single injection of
posttests were compared with each other with paired-samples t tests.
Data comparing the effects of experimental treatments in each of the 17
E2 or E2–BSA can acutely restore the appetitive component of
independent experiments were analyzed by two-way ANOVAs with
male sexual behavior in estrogen-deprived males. This suggests
treatments as the repeated factor and their order (two or three sub-
that the neural circuits underlying this aspect of the behavior are
groups) as the independent factor to exclude the possibility of long-term
significantly modulated by nongenomic actions of estrogens. The
effects of treatments that would interfere with the short-term effects
fact that E2 did not restore RCSM frequencies to the level dis-
under investigation. When significant, these ANOVAs were followed by
played by control animals suggests that some genomic priming
post hoc Fisher's least significant difference (LSD) tests, Newman–Keuls
by estrogens might additionally be necessary or alternatively that
tests, or Tukey's honestly significant difference tests (as appropriate de-
the acute treatment provided here was suboptimal. In contrast,
pending on the number of comparisons) comparing all conditions to
these data provide no information on whether estrogens acutely
influence copulatory behavior. Males chronically depleted of es-
All statistical analyses were performed with Statistica version 9
(StatSoft). Differences were considered significant for p ⬍ 0.05. All data
trogens showed no or very little copulatory behavior. Neural cir-
are expressed as mean ⫾ SEM.
cuits controlling this behavioral component may require a partialgenomic priming by estrogens before these steroids can exert any
acute action. This idea was tested in a second group of experi-
Effects of acute estrogen treatment in chronically estrogen-
ments by acutely blocking estrogen action or synthesis in males
deprived males (group 1)
exposed to the androgenic and estrogenic metabolites of T.
To assess behavioral effects of a rapid increase in estrogen bio-availability, CX males were chronically treated with SILASTIC T
Effects of blockade of local estrogen action or synthesis on
implants and received twice daily injections of the aromatase
sexually active males (group 2)
inhibitor VOR. They were thus exposed to the full range of an-
We next tested whether behavioral effects of endogenous estro-
drogenic effects of T, whereas genomic effects of estrogens were
gens produced by T aromatization in the brain would be rapidly
dramatically reduced. As predicted this
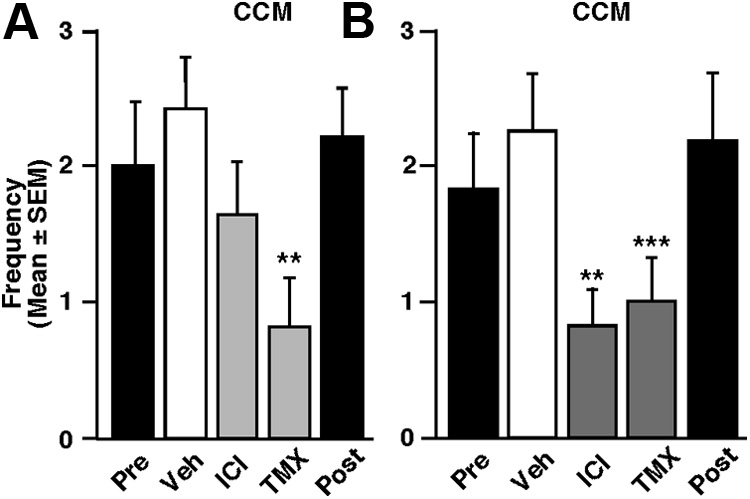
blocked by the estrogen receptor antagonists ICI and TMX. In
treatment nearly eliminated copulation
CX ⫹ T males displaying the full spectrum of male-typical sexual
The acute intracerebroventricular injection of 17-E2 (50 g)
behaviors, both antagonists significantly reduced RCSM fre-
to these animals resulted within 15 min in a doubling of RCSM
quencies within 15 min ; F
23.69, p ⬍ 0.001; ex-
frequency ; F
13.854, p ⫽ 0.004; experiment 1), yet
periment 4). This inhibition was even more pronounced when
170 • J. Neurosci., January 2, 2013 • 33(1):164 –174
Seredynski et al. • Acute Effects of Neuroestrogens on Behavior
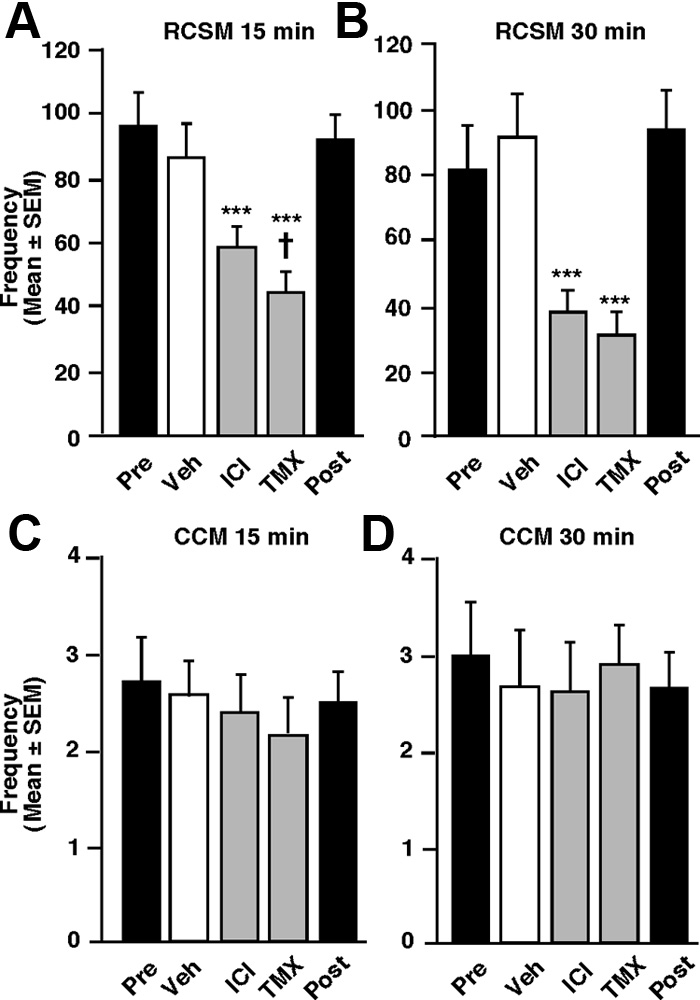
Figure 4.
Effects of acute blockade of estrogen receptors on the ASB and CSB of fully active
males (group 2). The blockade of estrogen action by the estrogen receptor antagonists ICI (50
g)andTMX(50g)acutelyinhibitsRCSMfrequency(A,B,n⫽15)butnotCCMfrequency(C,
D, n ⫽ 13 and 15, respectively) within 15 or 30 min compared with vehicle (Veh) injection
Figure 5.
Effects of acute deprivation in brain-derived estrogens on the ASB and CSB of fully
(white bars). The "Pre" and "Post" black bars provide reference behavior frequencies after
active males (group 2). Blockade of local estrogen synthesis by the aromatase inhibitors ATD (50
vehicle intracerebroventricular injections performed before and after the acute treatments, but
these data are not included in the statistical analyses. ***p ⬍ 0.001 versus vehicle; †p ⬍ 0.05
E (50 g; C) or its membrane-impermeant analog E – biotin (E -BIO, 50 g E equivalent; D)
versus ICI by Newman–Keuls post hoc tests after identification of a significant treatment effect
injected 15 min after VOR counteracts its effect on RCSM. The "Pre" and "Post" black bars provide
(repeated-measure) by two-way ANOVA.
reference behavior frequencies after vehicle intracerebroventricular injections performed be-fore and after the acute treatments, but these data are not included in the statistical analyses.
**p ⬍ 0.01 and ***p ⬍ 0.001 versus vehicle (Veh); †††p ⬍ 0.001 vs VOR by Newman–Keuls
tests were performed 30 min after the intracerebroventricular
post hoc tests after identification of a significant treatment effect (repeated measure, n ⫽ 12 in
injection ; F
24.705, p ⬍ 0.001; experiment 5). In
each case) by two-way ANOVA.
contrast, regardless of timing, these antagonists did not affect copu-latory behavior ,D; 15 min: F
1.122, p ⫽ 0.345, exper-
iment 6; 30 min: F
0.101, p ⫽ 0.903, experiment 7). The acute
iment 8), thus demonstrating that the suppression of estrogen
blockade of estrogen action thus significantly affected a measure of
production has the same effect as the blockade of its action and,
ASB but not its consummatory aspect, just as did the acute stimula-
notably, that the relevant estrogens are produced locally in the
tion by E2 in the first group of experiments.
brain. However, CSB was still unchanged by aromatase inhibitors
No order effect of the treatment was detected (F ⱕ 1.769, p ⱖ
0.161, p ⫽ 0.851; experiment 9).
0.212) except for RCSM tested after 30 min (F
11.02, p ⫽
No order effect (F ⱕ 0.438, p ⱖ 0.657) nor interaction was
0.010; baseline behavior was slightly different between sub-
detected in any of these experiments (F ⱕ 1.146, p ⱖ 0.366). In
groups). However, no interaction was detected in any of these
addition, none of the comparisons of the pretests and posttests
experiments (F ⱕ 1.695, p ⱖ 0.183). In addition, all comparisons
was significant (t ⱕ 0.168, p ⱖ 0.869). These results again indicate
of the pretests and posttests was not significant (t ⱕ 1.389, p ⱖ
that these effects are not associated with long-term actions of
0.190). Thus, these behavioral responses do not seem to be af-
fected by long-term effects of estrogens.
Importantly, the VOR-induced inhibition of ASB was almost
Nongenomic effects of estrogens are potentially mediated by
completely prevented by an E2 intracerebroventricular injection
different membrane receptors, including nuclear estrogen recep-
(50 or 100 g) administered 15 min after VOR ; 50 g:
tors of the ␣ or  subtype associated with the neuronal membrane
50.152, p ⬍ 0.001; experiment 10). Thus, the behavioral
but also G-protein-coupled
inhibition does not result from nonspecific adverse effects of the
receptors, such as GPR30
aromatase inhibitor but specifically from the depletion of endog-
and Gq-mER ICI and TMX block
enous estrogens. This rescue of VOR treatment effects by E2 was
some, but definitely not all, of these receptors. ASB and CSB
mimicked by another membrane-impermeable estrogen analog
could thus be acutely regulated by estrogens through different
2– biotin (50 g; ; F(2,18)
31.01, p ⬍ 0.001; experiment
receptors, thus explaining why these two aspects of behavior were
11), further confirming that rapid effects of E2 are initiated at the
differentially affected by these antagonists. To test this possibility,
membrane. Again, no order effect (F ⱕ 0.231, p ⱖ 0.797) was
birds were tested again but this time 30 min after an acute block-
detected in these experiments. No interaction was found after E2
ade of estrogen synthesis. A single injection of both the steroidal
injection (F
2.686, p ⫽ 0.064), but a significant interaction
(ATD) and nonsteroidal (VOR) aromatase inhibitors signifi-
was detected with E
2– biotin (F(4,18)
4.093, p ⫽ 0.015). This
cantly decreased ASB ; F
15.31, p ⬍ 0.001; exper-
interaction results from a decreased stimulation by E2– biotin in
Seredynski et al. • Acute Effects of Neuroestrogens on Behavior
J. Neurosci., January 2, 2013 • 33(1):164 –174 • 171
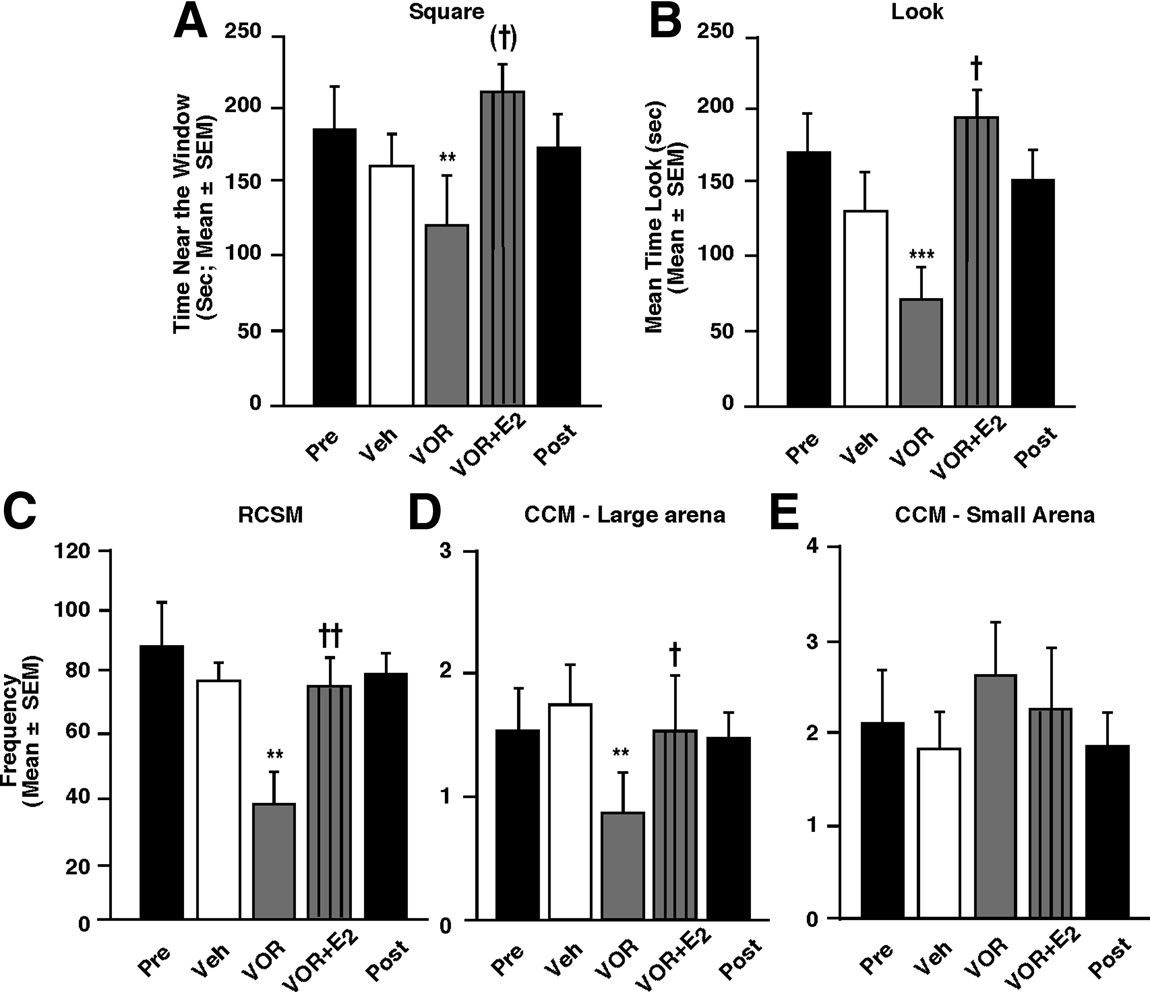
tion but not sexual performance. To assess this possibilityfurther, another group of CX ⫹ T males was used to test the acuteeffects of brain-derived estrogens on another measure of ASB, thelearned social proximity response. These animals were also usedto demonstrate the reproducibility of some of the results pre-sented previously.
As shown previously the animals pro-
gressively learned to stand in front of the window (from ⬃60 sduring the first test to 250 s of 300 s at the plateau) and spent anincreasing amount of time looking at the female (from ⬃45 sduring the first test to 200 s at the plateau). Thus, there was asignificant change in these behaviors across the eight acquisition
Figure 6.
Effects of acute blockade of estrogen action or synthesis on copulatory be-
tests (repetition effect: Square, F
12.530, p ⬍ 0.0001; Look,
havior of behaviorally active males tested in large test chambers (group 2). The acute
13.936, p ⬍ 0.0001). This response was then stable over
blockade of estrogen action by general estrogen antagonists (A, n ⫽ 14) or synthesis by
time even when tested in extinction as revealed by the com-
aromatase inhibitors (B, n ⫽ 12) profoundly inhibits CSB when measured in a large test
arena (90 ⫻ 90 ⫻ 50 cm) 30 min after injection compared with control vehicle (Veh)
parison of the time spent in the square and looking between
injection. The "Pre" and "Post" black bars provide reference behavior frequencies after
the last acquisition test and the first two extinction tests (rep-
vehicle intracerebroventricular injections performed before and after the acute treat-
etition effect: Square, F
2.213, p ⫽ 0.129; Look, F(2,26)
ments, but these data are not included in the statistical analyses. **p ⬍ 0.01 and ***p ⬍
2.328, p ⫽ 0.117).
0.001 versus vehicle (Veh) by Newman–Keuls post hoc tests after identification of a sig-
The learned social proximity response, as assessed by the time
nificant treatment effect (repeated measure) by two-way ANOVA.
spent near the window (square) and looking at the female, wasacutely reduced by aromatase inhibition, and this effect was com-pletely prevented by E
2 , B; Square, F(2,26)
4.867, p ⫽
the subgroup that received this treatment last (order in this sub-
0.016; Look, F
9.284, p ⬍ 0.001; experiment 14). No effect
group PG/VOR/VOR ⫹ E2– biotin). This decreased reaction can-
of the order of the treatments (Square, F
0.009, p ⫽ 0.991;
not be ascribed to lasting effects of the previous injection of VOR
0.114, p ⫽ 0.893) or their interaction with these
because a marked stimulation by E2– biotin was observed in the
treatments (Square, F
0.094, p ⫽ 0.983; Look, F(4,26)
subgroup exposed in sequence to VOR/VOR ⫹ E2– biotin/PG. In
0.284, p ⫽ 0.885) was noticed. In addition, the pretests and post-
addition, none of the comparisons of the pretests and posttests
tests did not differ either (t ⱕ 0.509, p ⱖ 0.618).
were found to be statistically significant (t ⱕ 0.219, p ⱖ 0.830).
The effects of these treatments (VOR and VOR ⫹ E2 com-
Overall, these findings support the independence of these effects
pared with PG) were then tested on the RCSM frequency, and
from long-term actions of estrogens.
copulatory behavior was assessed in the small and large test
One explanation for the striking discrepancy between rapid
chambers. These experiments fully confirmed the effects ob-
effects of estrogens on ASB and CSB could reside in the fact that
served in the second set of animals in the current study: changes
copulatory behavior was tested in a small arena in which motiva-
in brain-derived estrogens induced parallel changes in RCSM
tional decreases might not affect performance because both part-
frequency ; F
⫽ 13.965, p ⬍ 0.001; experiment 15), as
ners are in such close proximity that males reflexively perform the
well as in sexual performance when measured in a large arena in
copulatory sequence in the absence of sexual motivation; the
which the female can easily escape the male ; F
simple contact with the mate would trigger the performance of
the motor copulatory sequence. To test this hypothesis, effects of
7.429, p ⫽ 0.008; experiment 17) but not in the small arena in
estrogen receptor antagonists and aromatase inhibitors on CSB
which both partners are in very close proximity ; F(2,12)
were tested again but this time in a larger arena in which females
0.888, p ⫽ 0.437; experiment 16). Again, no order effect (F ⱕ
could more easily escape so that males had to actively pursue
0.864, p ⱖ 0.468) nor interaction (F ⱕ 2.279, p ⱖ 0.120) was
them to copulate successfully. In this new context, TMX, but not
detected in any of these experiments, and no difference was found
ICI, significantly inhibited copulation ; F
between the behavioral frequencies displayed during the pretests
p ⬍ 0.001; experiment 12). Furthermore, both aromatase inhib-
and posttests (t ⱕ 0.577, p ⱖ 0.579), with the exception of the
itors profoundly inhibited CSB ; F
RCSM test (t ⫽ 6.271, p ⬍ 0.0002; overall minor decrease in all
10.943, p ⬍
0.001; experiment 13). Thus, the acute blockade of estrogen ac-
subgroups over the course of the experiment).
tion affects CSB in contexts in which males must be sexually
Together, these results further demonstrate that acute changes
motivated and actively chase the female to mate.
in local estrogen bioavailability do not alter the ability of male
Again, the analyses did not reveal any order effect (F ⱕ2.367,
quail to produce a highly coordinated sexual motor response.
p ⱖ 0.149) nor interaction between treatments and their order
Indeed, the lack of effect in the small arena demonstrates that the
(F ⱕ 1.317, p ⱖ 0.294). In addition, none of the comparisons
absence of estrogens does not impair the ability of the male to
between the pretests and posttests was significant (t ⱕ 1.000, p ⱖ
mount and perform the highly stereotypical CCM. However,
0.338). Overall, this absence of order effects and of interaction
copulatory behavior can only be observed after the male has
between treatments and orders suggests that there is no carryover
searched for and approached a female. When examined in the
indicative of long-term effects of estrogens.
large arena, an absence of sexual performance thus indirectlyreflects a lack of motivation. That sexual performance is only
Effect of changes in brain-derived estrogens on the learned
affected by estrogens in the large arena clearly suggests that brain-
social proximity response in sexually active males (group 3)
derived estrogens rapidly affect sexual motivation but not perfor-
The results described so far suggested that the nongenomic ac-
mance as supported by the results obtained from two
tions of estrogens might preferentially control the sexual motiva-
independent measures of ASB.
172 • J. Neurosci., January 2, 2013 • 33(1):164 –174
Seredynski et al. • Acute Effects of Neuroestrogens on Behavior
Discussion
The present experiments show that
17-E2 significantly activates and estro-
gen receptor antagonists inhibit, with la-
tencies in the order of minutes, the
expression of behaviors reflecting sexual
motivation in male quail. Mechanistically,
these effects appear to be initiated at the
cell membrane level because these effects
aremimickedbytheinjectionofmembrane-
impermeable estrogens, such as E2–BSA and
E2–biotin. Finally, the observation that ASB
is blocked also within minutes by a single
intracerebroventricular injection of aroma-
tase inhibitors indicates that endogenous es-
trogens locally produced in the brain are
actually exerting these rapid controls of sex-
ual motivation.
The activation of appetitive and con-
summatory male sexual behavior by thegenomic action of brain-derived estro-gens has been extensively documentedacross
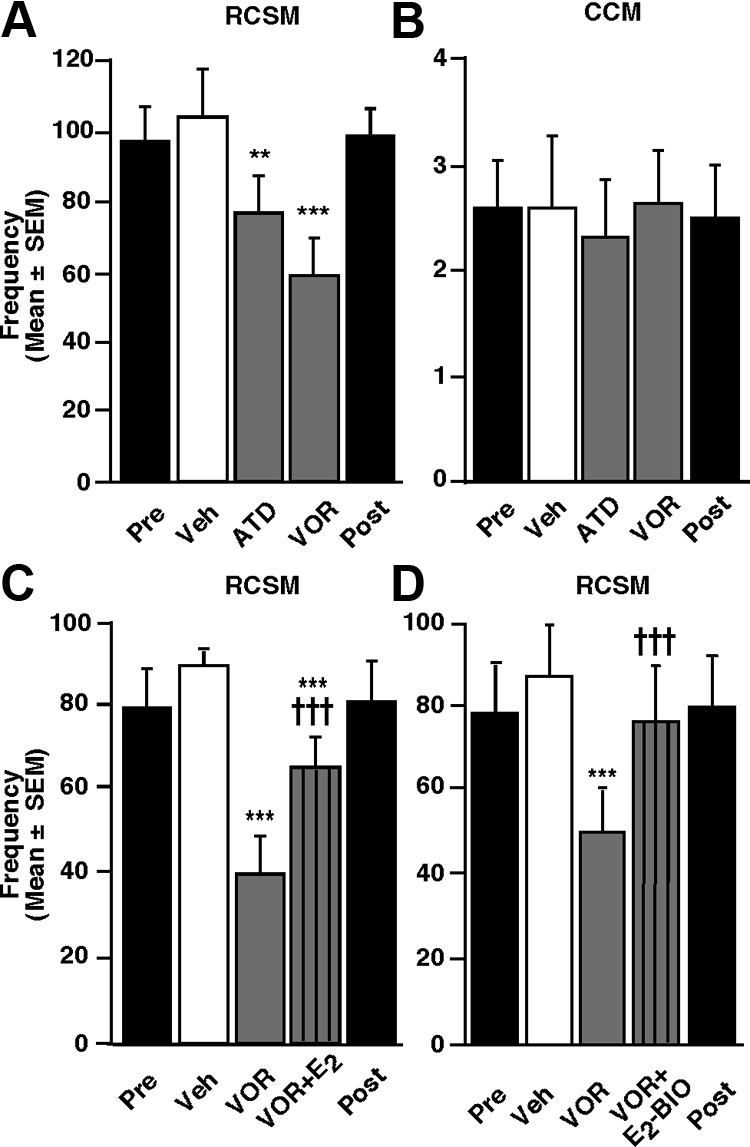
Figure 7.
Effects of acute blockade of brain estrogen synthesis associated or not with an acute E injection on appetitive and
consummatory aspects of male sexual behavior in sexually active males (group 3). Blockade of local estrogen synthesis by the
aromatase inhibitor VOR (50 g) acutely reduces whereas E (50 g) restores the time spent near the window (A) and looking at
the female (B) in the learned social proximity test (n ⫽ 16). Similar effects are observed on RCSM frequency (C, n ⫽ 9) and
specifically, the activation of copulatory
copulatory behavior assessed by the frequency of CCM measured in the large arena (D, n ⫽ 9). However, no such effects were
behavior and appetitive responses, such as
detected on copulatory behavior measured in the small arena (E, n ⫽ 9). The "Pre" and "Post" black bars provide reference
the female-induced increase in RCSM fre-
behavior frequencies after vehicle intracerebroventricular injections performed before and after the acute treatments. **p ⬍ 0.01
quency and the learned social proximity
and ***p ⬍ 0.001 versus vehicle (Veh); (†)p ⬍ 0.10, †p ⬍ 0.05, ††p ⬍ 0.01 versus VOR by Newman–Keuls post hoc tests after
response, requires local T aromatization
identification of a significant treatment effect (repeated measure) by two-way ANOVA.
in the preoptic area Recently, it had been suggested
Together, these data demonstrate that centrally administered
that estrogens also acutely control both behavioral components
17-E2 rapidly stimulates sexual motivation but not perfor-
with a more pronounced effect on the appetitive aspect of male-
mance (unless performance depends on motivation, as in the
typical sexual behavior in birds at least
tests performed in the large arena). Importantly, the basal (con-
trol) behavioral activity was remarkably stable across tests despite
these effects were highly variable. This might in part be explained
repeated treatments with various drugs. No effect of the order in
by the fact that all compounds were administered peripherally
which treatments were administered to different subgroups of
but also by the relative lack of previous genomic priming by the
birds could be generally detected, and there were usually no dif-
androgenic and/or estrogenic metabolites of T. Previous studies
ferences in behavioral frequencies between pretests and posttests,
have indeed tested whether acute administration of E
indicating that there is no long-term (presumably genomic)
store sexual behavior in CX rats or in
effect coupled to these acute treatments. Moreover, these
CX quail treated with a suboptimal dose of T whose effects
membrane-initiated effects are faster than the typical hours/days
were quite difficult to titrate The present
required for genomic actions. These data imply that the effects
design circumvented these problems by administering all
are independent of new transcription and rely on nongenomic
acute treatments directly in the brain (third ventricle) and
testing the effects of E
It was proposed recently that 17-E
2 either (1) in males chronically supplied
2 satisfies most, if not all,
with physiological T concentrations but chronically depleted
criteria needed to classify the steroid in the brain as a neuro-
in estrogens (group 1 of birds) or (2) in males chronically
modulator rather than a hormone
treated with T but acutely deprived of estrogens (groups 2 and
Among these criteria, the ability to exert
3). The present data demonstrate that brain-derived estrogens
rapid actions and the existence of rapid regulations of local syn-
can acutely facilitate a measure of ASB in the absence of
thesis were described in vitro, but clear evidence that E2 acts as a
genomic activation by estrogens (experiments 1 and 3), although
neuromodulator to alter behavioral responses was primarily
restoration seems to be more complete when genomic priming by
lacking. The remarkable reduction of sexual motivation observed
both androgenic and estrogenic metabolites is present and
here after central blockade of either estrogen action or synthesis
local aromatization is only inhibited acutely (experiments 10,
provides a clear example of rapid estrogenic effects on a complex
11, and 15). In contrast, the absence of effect of E
behavioral response. More importantly, it provides direct evi-
inhibited copulatory behavior of males chronically deprived
dence that the estrogens relevant for this rapid control are pro-
of estrogens (experiment 2) indicates that the neural circuits
duced locally. Because membrane receptors underlying these
underlying the control of the copulatory sequence critically
effects are probably available continuously, local E2 concentra-
depend on the genomic activation by estrogens.
tion must vary rapidly to control these on/off switches in moti-
Seredynski et al. • Acute Effects of Neuroestrogens on Behavior
J. Neurosci., January 2, 2013 • 33(1):164 –174 • 173
vation. This conclusion provides a functional significance to the
changes are mediated by classical neurotransmitters, we demon-
rapid fluctuations in aromatase activity described recently in
strate here that the rapid on/off switches in sexual motivation are
brain areas controlling sexual behavior
controlled, at least in part, by brain-derived estrogens. These re-
sults indicate that the same molecule has evolved mechanisms to
It has been demonstrated that preoptic aromatase activity is
control different aspects of behavior in distinct temporal do-
very rapidly inhibited in the male quail brain after sexual inter-
mains. Given the pleiotropic effects of estrogens, one may won-
actions with a female Somewhat
der whether other responses associated with reproduction (e.g.,
surprisingly, however, this enzymatic decrease was already ob-
territoriality, parenting) also depend on a slow genomic regula-
served after only visual exposure to the female without the male
tion of the neuronal plasticity underlying behavioral activation
having had an opportunity to copulate with her
and an acute control of motivation to engage in behavior. Ex-
This decrease is therefore
tending this duality to non-reproductive responses, such as
not the direct result of engaging in copulation per se. Based on
changes in cognition or neuroprotection, might have profound
this observation, it was hypothesized that the high aromatase
activity and the resulting elevated local concentration of estro-gens might be critical for promoting sexual motivation and ap-
petitive behavior through which the male searches for and courts
Adkins EK, Adler NT (1972) Hormonal control of behavior in the Japanese
the female. Copulatory performance sensu stricto would then no
quail. J Comp Physiol Psychol 81:27–36.
longer depend on these rapid nongenomic effects of estrogens
Bakker J, Honda S, Harada N, Balthazart J (2004) Restoration of male sexual
but rather on the action of other neurotransmitters, such as do-
behavior by adult exogenous estrogens in male aromatase knockout mice.
pamine, that have been primed and/or enhanced by modifica-
Horm Behav 46:1–10.
tions of brain neurochemistry resulting from the transcriptional
Ball GF, Balthazart J (2010) Japanese quail as a model system for studying
the neuroendocrine control of reproductive and social behaviors. ILAR J
effects of estrogens (for a detailed discussion, see
This interpretation fits well with the present results
Balthazart J, Ball GF (2006) Is brain estradiol a hormone or a neurotransmit-
demonstrating that 17-E2 activates within minutes appetitive
ter? Trends Neurosci 29:241–249.
aspects of male sexual behavior in quail but that the activation of
Balthazart J, Foidart A, Hendrick JC (1990a) The induction by testosterone
copulatory performance sensu stricto requires a more sustained
of aromatase activity in the preoptic area and activation of copulatory
stimulation by estrogens and is not affected by these steroids on a
behavior. Physiol Behav 47:83–94.
short-term timescale. Evidence coming from radioenzyme assays
Balthazart J, Evrard L, Surlemont C (1990b) Effects of the non-steroidal
inhibitor R76713 on testosterone-induced sexual behavior in the Japanese
of aromatase activity after sexual interactions
quail (Coturnix coturnix japonica). Horm Behav 24:510 –531.
and from behavioral effects
of intracerebroventricular pharmacological treatments (present
Balthazart J, Reid J, Absil P, Foidart A, Ball GF (1995) Appetitive as well as
study) thus converges to indicate that estrogens independently
consummatory aspects of male sexual behavior in quail are activated by
modulate appetitive and consummatory aspects of male sexual
androgens and estrogens. Behav Neurosci 109:485–501.
behavior by nongenomic and genomic mechanisms, respectively.
Balthazart J, Castagna C, Ball GF (1997) Aromatase inhibition blocks the
Developing and maintaining signaling mechanisms that op-
activation and sexual differentiation of appetitive male sexual behavior in
erate in distinct temporal domains on an integrated set of behav-
japanese quail. Behav Neurosci 111:381–397.
ioral responses may appear as a waste of resources. That these two
Balthazart J, Absil P, Ge´rard M, Appeltants D, Ball GF (1998) Appetitive and
modes of action regulate distinct behavioral components (moti-
consummatory male sexual behavior in japanese quail are differentialy
vation vs performance), however, makes good sense when con-
regulated by subregions of the preoptic medial nucleus. J Neurosci 18:
sidering the natural constraints associated with reproduction. In
Balthazart J, Arnold AP, Adkins-Regan E (2009a) Sexual differentiation of
the temperate zone, reproduction is synchronized with environ-
brain and behavior in birds. In: Hormones, brain and behavior, Ed 2
mental cues (e.g., day length, temperature, food availability, etc.)
(Pfaff DW, Arnold AP, Etgen AM, Fahrbach SE, Rubin RT, eds), pp
to limit reproductive efforts to seasonal time periods that are
1745–1787. San Diego: Academic.
most favorable to mate and raise offspring. Seasonal changes in
Balthazart J, Taziaux M, Holloway K, Ball GF, Cornil CA (2009b) Behav-
circulating hormones prepare the organisms for breeding (e.g.,
ioral effects of brain-derived estrogens in birds. Ann N Y Acad Sci 1163:
maturation of the reproductive tract, development of sexually
dimorphic traits, etc). These physiological changes include a pro-
Charlier TD, Harada N, Balthazart J, Cornil CA (2011) Human and quail
aromatase activity is rapidly and reversibly inhibited by phosphorylating
found reorganization of the neuronal circuitry that controls the
conditions. Endocrinology 152:4199 – 4210.
performance of coordinated motor sequences in response to sex-
Christensen LW, Clemens LG (1974) Intrahypothalamic implants of testos-
ual stimuli These processes
terone or estradiol and resumption of masculine sexual behavior in long-
involving extensive protein synthesis, neurogenesis, and axonal
term castrated male rats. Endocrinology 95:984 –990.
growth require time. The time course of estrogen transcriptional
Christensen LW, Clemens LG (1975) Blockade of testosterone-induced
activity is well suited to address these needs within the time frame
mounting behavior in the male rat with intracranial application of thearomatization inhibitor, androst-1,4,6,-triene-3,17-dione. Endocrinol-
required for seasonal effects. However, on a shorter timescale,
ogy 97:1545–1551.
even when the reproductive system is fully mature, sexual activity
Cornil CA, Ball GF (2010) Effects of social experience on subsequent sexual
is not observed constantly. Instead, animals display bouts of sex-
performance in naive male Japanese quail (Coturnix japonica). Horm
ual activity alternating with other activities required to satisfy
Behav 57:515–522.
other needs (e.g., feeding, grooming, etc.). They also interrupt
Cornil CA, Holloway KS, Taziaux M, Balthazart J (2004) The effects of
mating when it would be inappropriate or even dangerous (e.g.,
aromatase inhibition on testosterone-dependent conditioned rhyth-mic cloacal sphincter movement in male Japanese quail. Physiol Behav
during a storm, in the presence of a predator or a competitor).
Mechanisms have evolved to adapt organisms to these internal or
Cornil CA, Dejace C, Ball GF, Balthazart J (2005a) Dopamine modulates
external cues and acutely control the drive to engage in sexual
male sexual behavior in Japanese quail in part via actions on noradrener-
behavior. Although it is often assumed that acute behavioral
gic receptors. Behav Brain Res 163:42–57.
174 • J. Neurosci., January 2, 2013 • 33(1):164 –174
Seredynski et al. • Acute Effects of Neuroestrogens on Behavior
Cornil CA, Dalla C, Papadopoulou-Daifoti Z, Baillien M, Dejace C, Balthaz-
Qiu J, Bosch MA, Tobias SC, Grandy DK, Scanlan TS, Ronnekleiv OK, Kelly
art J (2005b) Sexual behavior affects preoptic aromatase activity and
MJ (2003) Rapid signaling of estrogen in hypothalamic neurons involves
brain monoamines' levels. Endocrinology 146:3809 –3820.
a novel G-protein-coupled estrogen receptor that activates protein kinase
Cornil CA, Dalla C, Papadopoulou-Daifoti Z, Baillien M, Balthazart J
C. J Neurosci 23:9529 –9540.
(2006a) Estradiol rapidly activates male sexual behavior and affects brain
Remage-Healey L, Joshi NR (2012) Changing neuroestrogens within the
monoamine levels in the quail brain. Behav Brain Res 66:110 –123.
auditory forebrain rapidly transform stimulus selectivity in a downstream
sensorimotor nucleus. J Neurosci 32:8231– 8241.
Cornil CA, Taziaux M, Baillien M, Ball GF, Balthazart J (2006b) Rapid ef-
Remage-Healey L, Coleman MJ, Oyama RK, Schlinger BA (2010) Brain es-
fects of aromatase inhibition on male reproductive behaviors in Japanese
trogens rapidly strengthen auditory encoding and guide song preference
quail. Horm Behav 49:45– 67.
in a songbird. Proc Natl Acad Sci U S A 107:3852–3857.
Cornil CA, Ball GF, Balthazart J, Charlier TD (2011) Organizing effects of
Remage-Healey L, Maidment NT, Schlinger BA (2008) Forebrain steroid
sex steroids on brain aromatase activity in quail. PLoS One 6:e19196.
levels fluctuate rapidly during social interactions. Nat Neurosci 11:1327–
Cornil CA, Ball GF, Balthazart J (2012) Rapid control of male typical behav-
Revankar CM, Cimino DF, Sklar LA, Arterburn JB, Prossnitz ER (2005) A
iors by brain-derived estrogens. Front Neuroendocrinol 33:425– 446.
transmembrane intracellular estrogen receptor mediates rapid cell signal-
ing. Science 307:1625–1630.
Cross E, Roselli CE (1999) 17-estradiol rapidly facilitates chemoinves-
Riters LV, Absil P, Balthazart J (1998) Effects of brain testosterone implants
tigation and mounting in castrated male rats. Am J Physiol 276:
on appetitive and consummatory components of male sexual behavior in
Japanese quail. Brain Res Bull 47:69 –79.
de Bournonville C, Dickens MJ, Ball GF, Balthazart J, Cornil CA (2012)
Robinson JE, Follett BK (1982) Photoperiodism in Japanese quail: the ter-
Dynamic changes in brain aromatase activity following sexual interac-
mination of seasonal breeding by photorefractoriness. Proc R Soc Lond B
tions: where, when and why? Psychoneuroendocrinology. Advance on-
Biol Sci 215:95–116.
line publication. doi:10.1016/j.psyneuen.2012.09.001.
Roepke TA, Qiu J, Bosch MA, Rønnekleiv OK, Kelly MJ (2009) Cross-talk
Delville Y, Hendrick JC, Sulon J, Balthazart J (1984) Testosterone metabo-
between membrane-initiated and nuclear-initiated oestrogen signalling
lism and testosterone-dependent characteristics in Japanese quail. Physiol
in the hypothalamus. J Neuroendocrinol 21:263–270.
Behav 33:817– 823.
Roselli CE, Horton LE, Resko JA (1985) Distribution and regulation of aro-
Dewing P, Boulware MI, Sinchak K, Christensen A, Mermelstein PG,
matase activity in the rat hypothalamus and limbic system. Endocrinology
Micevych P (2007) Membrane estrogen receptor-alpha interactions
117:2471–2477.
with metabotropic glutamate receptor 1a modulate female sexual recep-
Sachs BD (1967) Photoperiodic control of the cloacal gland of the Japanese
tivity in rats. J Neurosci 27:9294 –9300.
quail. Science 157:201–203.
Dickens MJ, Cornil CA, Balthazart J (2011) Acute stress differentially affects
Saldanha CJ, Remage-Healey L, Schlinger BA (2011) Synaptocrine signaling:
aromatase activity in specific brain nuclei of adult male and female quail.
steroid synthesis and action at the synapse. Endocr Rev 32:532–549.
Endocrinology 152:4242– 4251.
Domjan M, Hall S (1986) Determinants of social proximity in Japanese
Santen RJ, Brodie H, Simpson ER, Siiteri PK, Brodie A (2009) History of
quail (Coturnix coturnix japonica): male behavior. J Comp Psychol 100:
aromatase: saga of an important biological mediator and therapeutic tar-
get. Endocr Rev 30:343–375.
Domjan M, Akins C, Vandergriff DH (1992) Increased responding to fe-
Seiwert CM, Adkins-Regan E (1998) The foam production system of the
male stimuli as a result of sexual experience: tests of mechanisms of learn-
male Japanese quail: characterization of structure and function. Brain
ing. Q J Exp Psychol 45B:139 –157.
Behav Evol 52:61– 80.
Etgen AM, Pfaff DW (2009) Molecular mechanisms of hormone action on
Stevis PE, Deecher DC, Suhadolnik L, Mallis LM, Frail DE (1999) Differen-
behavior. San Diego: Academic.
tial effects of estradiol and estradiol-BSA conjugates. Endocrinology 140:
Germain PS, Metezeau P, Tiefenauer LX, Kiefer H, Ratinaud MH, Habrioux
G (1993) Use of a biotinyl-estradiol derivative to demonstrate estradiol-
Taziaux M, Cornil CA, Balthazart J (2004) Aromatase inhibition blocks the
membrane binding sites on adherent human breast cancer MCF-7 cells.
expression of sexually-motivated cloacal gland movements in male quail.
Anticancer Res 13:2347–2353.
Behav Processes 67:461– 469.
Holloway KS, Balthazart J, Cornil CA (2005) Androgen mediation of con-
Taziaux M, Keller M, Bakker J, Balthazart J (2007) Sexual behavior activity
ditioned rhythmic cloacal sphincter movements in Japanese quail (Cotur-
tracks rapid changes in brain estrogen concentrations. J Neurosci 27:
nix japonica). J Comp Psychol 119:49 –57.
Hutchison JB, Steimer TJ, Hutchison RE (1986) Formation of behaviorally
Thompson RR, Goodson JL, Ruscio MG, Adkins-Regan E (1998) Role of the
active estrogen in the dove brain: induction of preoptic aromatase by
archistriatum nucleus taeniae in the sexual behavior of male Japanese
intracranial testosterone. Neuroendocrinology 43:416 – 427.
quail (Coturnix japonica): a comparison of function with the medial nu-
cleus of the amygdala in mammals. Brain Behav Evol 51:215–229.
Hutchison RE (1978) Hormonal differentiation of sexual behavior in Japa-
nese quail. Horm Behav 11:363–387.
Tramontin AD, Brenowitz EA (2000) Seasonal plasticity in the adult brain.
Kleitz-Nelson HK, Cornil CA, Balthazart J, Ball GF (2010) Differential ef-
Trends Neurosci 23:251–258.
fects of central injections of D1 and D2 receptor agonists and antagonists
Tremere LA, Pinaud R (2011) Brain-generated estradiol drives long-term
on male sexual behavior in Japanese quail. Eur J Neurosci 32:118 –129.
optimization of auditory coding to enhance the discrimination of com-
munication signals. J Neurosci 31:3271–3289.
Kow LM, Pfaff DW (2004) The membrane actions of estrogens can poten-
Tremere LA, Jeong JK, Pinaud R (2009) Estradiol shapes auditory process-
tiate their lordosis behavior-facilitating genomic actions. Proc Natl Acad
ing in the adult brain by regulating inhibitory transmission and plasticity-
Sci U S A 101:12354 –12357.
associated gene expression. J Neurosci 29:5949 –5963.
Micevych P, Dominguez R (2009) Membrane estradiol signaling in the
Tsai MJ, O'Malley BW (1994) Molecular mechanisms of action of steroid/
brain. Front Neuroendocrinol 30:315–327.
thyroid receptor superfamily members. Annu Rev Biochem 63:451– 486.
Naftolin F, Ryan KJ, Davies IJ, Reddy VV, Flores F, Petro Z, Kuhn M, White
RJ, Takaoka Y, Wolin L (1975) The formation of estrogens by central
Vagell ME, McGinnis MY (1997) The role of aromatization in the restora-
neuroendocrine tissues. Rec Prog Horm Res 31:295–319.
tion of male rat reproductive behavior. J Neuroendocrinol 9:415– 421.
Prossnitz ER, Sklar LA, Oprea TI, Arterburn JB (2008) GPR30: a novel ther-
apeutic target in estrogen-related disease. Trends Pharmacol Sci 29:116 –
Woolley CS (2007) Acute effects of estrogen on neuronal physiology. Annu
Rev Pharmacol Toxicol 47:657– 680.
Source: http://humanbehaviors.free.fr/References%20-%20Articles/Neuroestrogens%20rapidly%20regulate%20sexual%20motivation%20but%20not%20performance.pdf
Project appraisal document
FOR OFFICIAL USE ONLY Report No: PAD614 INTERNATIONAL DEVELOPMENT ASSOCIATION PROJECT APPRAISAL DOCUMENT PROPOSED GLOBAL PARTNERSHIP FOR EDUCATION GRANT IN THE AMOUNT OF US$100 MILLION REPUBLIC OF UGANDA TEACHER AND SCHOOL EFFECTIVENESS PROJECT Education Global Practice Eastern Africa Country Cluster 1, AFCE1 Africa Region This document has a restricted distribution and may be used by recipients only in the performance of their official duties. Its contents may not otherwise be disclosed without World Bank authorization.
Microsoft word - pharmaceutics8
Bulletin of Pharmaceutical Research 2012;1(S):41 An Official Publication of Association of Pharmacy Professionals ISSN: 2249-6041 (Print); ISSN: 2249-9245 (Online) GASTRORETENTIVE TARGETING TECHNOLOGY FOR ERADICATION OF HELICOBACTER PYLORI INFECTION Rakesh Pahwa*, Lovely Chhabra, Arunima Nath, Vipin Kumar Dept. of Pharmaceutics, Institute of Pharmaceutical Sciences, Kurukshetra University, Kurukshetra, Haryana