Curis.ku.dk
University of CopenhagenAcid-base transport in pancreas-new challenges
Novak, Ivana; Haanes, Kristian Agmund; Wang, Jing Published in:Frontiers in Physiology Document VersionPublisher's PDF, also known as Version of record Citation for published version (APA):Novak, I., Haanes, K. A., & Wang, J. (2013). Acid-base transport in pancreas-new challenges. Frontiers inPhysiology, 4, [380]. DOI: 10.3389/fphys.2013.00380 Download date: 07. Oct. 2016 *, Kristian A. Haanes † and Jing Wang †
Department of Biology, University of Copenhagen, Copenhagen, Denmark Along the gastrointestinal tract a number of epithelia contribute with acid or basic Ebbe Boedtkjer, Aarhus University, secretions in order to aid digestive processes. The stomach and pancreas are the most extreme examples of acid (H+) and base (HCO−) transporters, respectively. Nevertheless, they share the same challenges of transporting acid and bases across epithelia and Martin Diener, University Giessen, effectively regulating their intracellular pH. In this review, we will make use of comparative GermanyUrsula E. Seidler, Hannover Medical physiology to enlighten the cellular mechanisms of pancreatic HCO− and fluid secretion, School, Germany which is still challenging physiologists. Some of the novel transporters to consider in pancreas are the proton pumps (H+-K+-ATPases), as well as the calcium-activated K+ and Ivana Novak, Molecular Integrative Cl− channels, such as KCa3.1 and TMEM16A/ANO1. Local regulators, such as purinergic Physiology, Department of Biology,University of Copenhagen, August signaling, fine-tune, and coordinate pancreatic secretion. Lastly, we speculate whether Krogh Building, Universitetsparken dys-regulation of acid-base transport contributes to pancreatic diseases including cystic 13, Copenhagen Ø, DK 2100, fibrosis, pancreatitis, and cancer.
Denmarke-mail: Keywords: bicarbonate transport, proton transport, H+-K+-ATPase, KCa3.1, IK, TMEM16A, ANO1, pancreatic duct
†Present address:
Kristian A. Haanes, Department of
Clinical Experimental Research,
Glostrup Research Institute,
Copenhagen University Hospital,
Glostrup, Denmark;
Jing Wang, National Institute for
Viral Disease Control and
Prevention, Chinese Center for
Disease Control and Prevention,
Beijing, China
INTRODUCTION: ACID-BASE FLUXES ALONG THE
). In the intestinal phase of digestion, pancreatic ducts secrete In multicellular organisms the digestive system exhibits marked HCO−-rich fluid that contributes to alkalinization of acid chyme acid/base segmentation and gradients across the epithelia. The in duodenum. The acid generated is then transported toward most extreme examples of the acid/base transporters are the the interstitium, and one would expect an acid tide, depending stomach and the pancreas, which conduct a vectorial transport on ingested food and passage through the stomach (Rune and of acid/base to one side and base/acid to the other side of the epithelium In the stomach, the parietal cells of the From these simple considerations several questions arise. Do pyloric glands secrete H+ toward lumen (HCl), leaving HCO− the stomach and pancreas epithelia have some transport mecha- to be transported into the interstitium and blood. Thus, the nisms in common, or do they solve the task of acid-base transport phenomenon of the alkaline tide, i.e., higher blood pH in con- in different ways? nection with digestion, is well known as part of the post-prandial The molecular mechanism and regulation of stomach acid gastric phase secretion, which in humans is relatively small com- secretion is well established. In short, it involves gastric H+-K+- pared to animals that ingest large amounts of food at one time ATPases comprising of α1 and β subunits coded by ATP4A andATP4B genes. These pumps are present in tubulovesicles of pari- Abbreviations: BK, big conductance K+ channel, also named KCa1.1 and maxi-
etal cells and delivered to the luminal membranes in conjunction K+, coded by KCNMA1; CaCC, Ca2+-activated Cl− channel, e.g., TMEM16A with specific K+ (KCNQ1, KCNJ15, KCNJ10) and Cl− channels also known as ANO1; CA, carbonic anhydrase; CCK, cholecystokinin, CF, cysticfibrosis; [Ca2+]i, intracellular Ca2+ activity; CFTR, the cystic fibrosis transmem- (CFTR, CLIC-6, SCL26A9), and thereby resulting in HCl secre- brane conductace regulator; EBIO, 1-ethyl-2-benzimidazolinone; GK , conductance for K+; H+-K+-ATPases or pumps, colonic type (coded by ATP12A) and gastric ). Gastric acid secretion is regulated by neural, hormonal, types (coded by ATP4A and ATP4B); IK, intermediate conductance K+ channel, paracrine and chemical stimuli, e.g., acetylcholine, gastrin, ghre- also named KCa3.1; IRBIT, inositol 1,4,5-triphosphate (InsP3) receptor-bindingprotein released with InsP3; NBCe1 or pNBC, electrogenic Na+-HCO− trans- lin, histamine. As a protection against strong acid and pepsins, porter; NBCn1, electroneutral Na+-HCO3- transporter; NHE, Na+/H+ exchanger; the surface epithelium secretes HCO−, mucus and other fac- NKCC1, Na+-K+-2Cl− cotransporter; PKA, protein kinase A; PKC, proteins kinase tors, forming gastric diffusion barrier The validity C; SLC26A6, electrogenic Cl−-/2HCO−- exchanger; VNUT, vesicular nucleotide transporter, SLC17A9; V-H+-pump, vacuolar type H+-ATPase; ZG, zymogen of the model is confirmed by well-used drugs, including proton pump inhibitors and H2-histamine receptor blockers, to curb the
Novak et al.
Acid-base transport in pancreas
the latter two, which are expressed in pancreas (see below), othercandidates remain to be explored.
Pancreatic ducts comprise 5–20% of the tissue mass, depend-
ing on the species; morphologically they are different - progress-ing from flat centroacinar cells, cuboidal cells in intercalated, andsmall intralobular ducts to columnar heterogenous cells lininglarger distal ducts Bouwensand Pipeleers, ). At large, it is accepted that pancreatic ductssecrete isotonic NaHCO3 rich fluid. However, the concentrationof HCO− is not constant; it decreases with secretory rates—a
pattern that is mirrored by Cl−. The HCO− excretory pat-
terns are remarkably similar between various species, providingthat secretory rates are corrected for the duct mass In early studies it was proposedthat pancreatic secretion and ionic composition is a two stageprocess—primary secretion and ductal modification, the so calledadmixture hypothesis. Another, the exchange theory, also namedthe salvage mechanism, states that at lower secretory rates duc-tal transporters are presumably not saturated and therefore, are
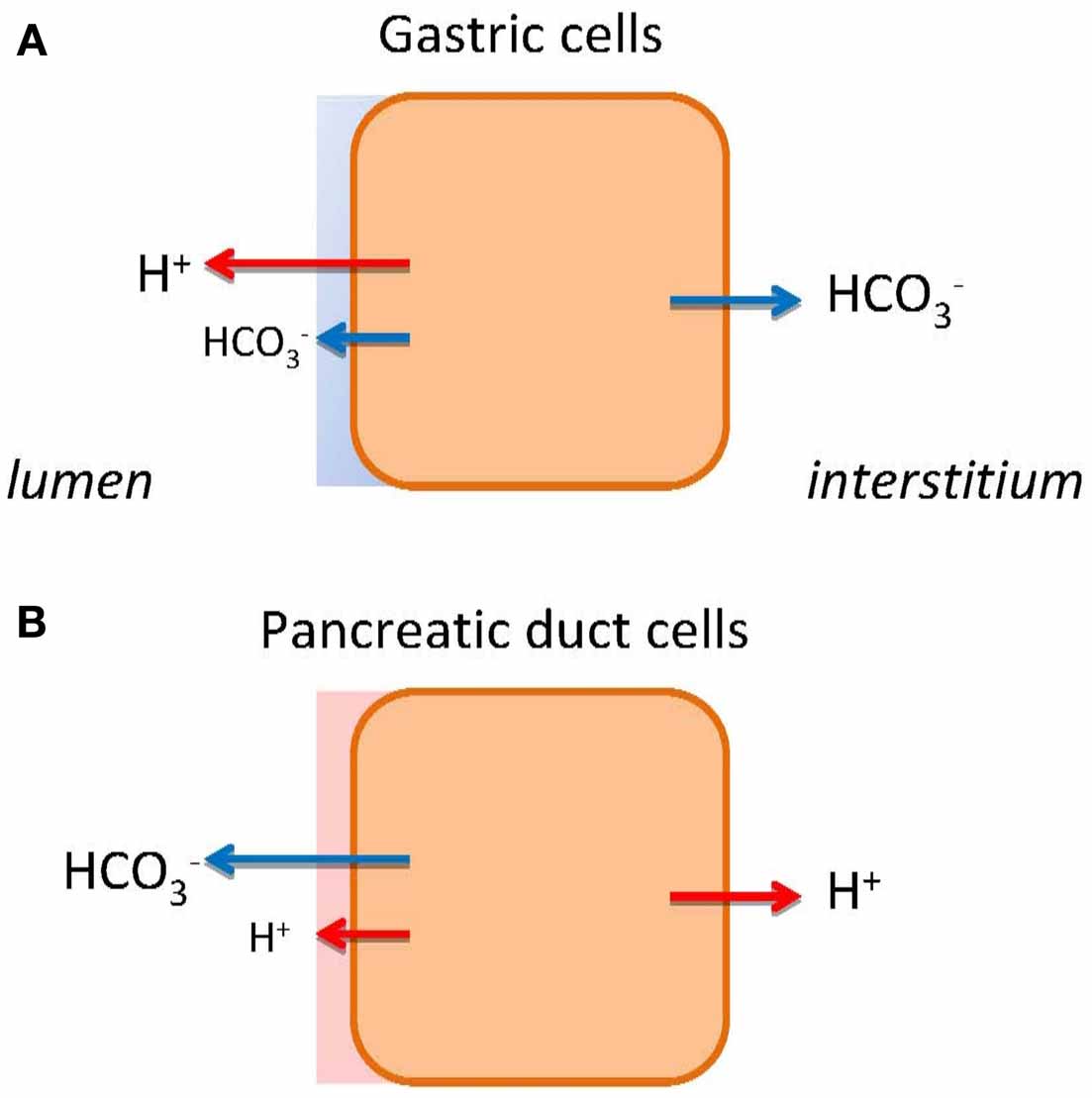
FIGURE 1 HCO− and H+ transport in gastric cells (A) and pancreatic
capable of exchanging luminal HCO− for interstitial Cl−. This
duct cells (B). The models show schematically different types of epithelia
exchange phenomenon was first demonstrated on the main cat
as single cells. The transport of H+ or HCO− to the bulk luminal fluid is
duct . The third explanation, regarding vary-
shown with large arrows. The small arrows on luminal side indicate HCO−
ing HCO− concentrations, pertains H+ secretion from acini (see
and H+ secretions to the mucosal buffer zone. Flux of HCO− and H+ to the
above) or ducts (see below).
interstititum/blood side indicates expected alkaline or acid tides.
NOVEL ION CHANNELS AND PUMPS CONTRIBUTING TO
peptic and duodenal ulcers and reflux diseases ).
ACID-BASE TRANSPORT IN PANCREATIC DUCTS
In contrast, we do not understand the mechanism behind pan-
The ion transport models for pancreatic ducts have been
creatic alkaline (HCO−) secretion fully. Therefore, therapeutic
described in several recent reviews Steward
intervention is not possible, e.g., for cystic fibrosis patients.
and Ishiguro, ). The outline of the model is given in The
PANCREATIC SECRETION—CONTRIBUTION FROM ACINI
following sections will focus on novel additions to the model.
AND DUCTS
Pancreas is composed of two main types of epithelia—secretory
PROTON PUMPS
acini and excretory ducts. Acini have relatively uniform mor-
Ion channels and transporters proposed in the classical model
phology. They secrete digestive enzymes, NaCl-rich fluid and
for HCO− secretion rely on gradients created by the Na+/K+-
various factors that contribute to signaling in down-stream ducts.
ATPase . However, we cannot explain formation of
Studies on normal human and rodent pancreas, stimulated by
high HCO− concentrations and the fact that inhibitors of NHE1,
predominantly acinar agonists, e.g., cholecytokinin (CCK), result
NBC (and NKCC1), and CA are relatively ineffective in blocking
in neutral or weakly alkaline pancreatic juice
However, a recent
One solution is that a primary pump could be involved, such as
study using acinar preparation and bioimaging techniques shows
the vacuolar type H+-ATPase (V-H+-pump), to pump H+ out
that acinar secretion is acidic due to acidic zymogen granules
to interstitium and leave HCO− for the luminal transport. In
(ZG) although acidity of mature ZG
one study, such vacuolar H+ pump on the basolateral membrane
has been discussed
was proposed and detected immunohisto-
). Nevertheless, a potential acid load from acini challenging
chemically Several functional studies gave
proximal ducts has been considered ). One
contradictory findings de
possible defense mechanism could be activation of ducts by aci-
Ondarza and Hootman, ). Taking an inspiration from gas-
nar agonist; generally this seems not to be the case. Alternatively,
tric glands, the colon and kidney distal tubules, we considered
paracrine agonists such as ATP released by acini could stimulate
whether pancreatic ducts express H+-K+-ATPases. Indeed, we
ducts by purinergic signaling
found that rodent ducts express both the gastric and non-gastric
). Lastly, pancreatic ducts might have ability to sense and
(colonic) types H+-K+-ATPases Inhibition
react to acid/base locally. There are a number of acid/base sen-
of these with proton pump inhibitors reduced pHi recovery in
sors at the single cell and whole organ level
response to acid loads; more importantly, they reduced secretion
in isolated pancreatic ducts. Thus, these functional studies sup-
acid sensitive ASIC and TRP channels, HCO− sensitive adenylate
port the theory that pancreatic ducts resemble gastric glands—
cyclase, pH-sensitive K+ channels, and P2X receptors. Except for
just working in reverse, expelling H+ toward the blood side
Novak et al.
Acid-base transport in pancreas
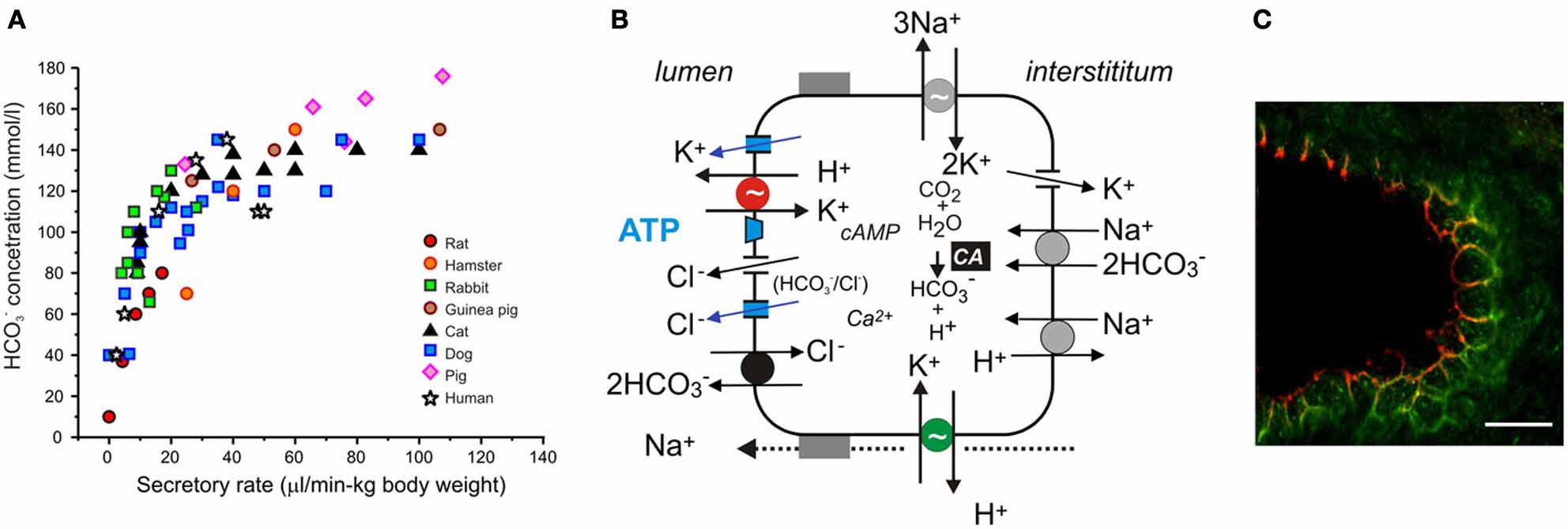
FIGURE 2 Acid/base transport in pancreas. (A) The relation between
certain conditions, through Cl− channels. The luminal Cl− channels are CFTR
secretory rates and HCO− concentrations in pancreatic juice of various
and TMEM16A (see text). There are a number of K+ channels expressed on
species. Secretions were stimulated by secretin and secretory rates were
the luminal and basolateral membranes, e.g., KCa3.1, KCa1.1, KCNQ1 (see
corrected for body weights. (B) The model of ion transport in a secreting
text). The luminal and basolateral H+-K+-ATPases are indicated in red and
pancreatic duct cell with novel transporters, channels and luminal purinergic
green, and supposedly contribute to the luminal buffer zone and the H+ efflux
signaling and receptors indicated in color and discussed in the review.
to intersititum, respectively. Other ion channels and transporters, such as
Intracellular HCO− is derived from CO
NHE3, SLC26A3, NBC3, NKCC1, and aquaporins have a differential
2 through the action of carbonic
anhydrase (CA) and from HCO− uptake via the electrogenic Na+–HCO−
distribution in the duct tree and for simplicity are not included in the model.
cotransporter (pNBC, NBCe1). H+ is extruded at basolateral membrane by
(C) Immunolocalization of the gastric (red) and non-gastric (green) H+-K+
the Na+/H+ exchanger (NHE1). HCO− efflux across the luminal membrane is
pumps in rat pancreatic duct. The bar is 20 μm. Modified from
mediated by the electrogenic Cl−/HCO− exchanger (SLC26A6), and under
and leaving HCO− for the luminal transport The
is—these luminal pumps are safeguarding luminal cell surface
immunohistochemical study showed that the H+-K+-ATPases
with acid secretions to protect against the bulk alkaline secre-
(mainly colonic type) are localized to the basolateral membrane
tions, which at pH >8 would be caustic to cells. Thus, pancreatic
ducts would have protective buffer (and mucus) zone, which is
However, H+-K+-ATPases, especially the gastric form, are
reminiscent to the buffer zone in the stomach, though achieved
also localized at or close to the luminal membrane
by H+ rather than HCO− secretion In addition,
). It seems counterintuitive to place H+ pumps
the luminal H+-K+ pumps would recirculate K+ extruded by the
on the HCO− secreting luminal membrane. Nevertheless, there
luminal K+ channels Lastly, luminal H+-K+ pumps
are epithelia that are high HCO− secretors and yet express H+
in distal ducts would by virtue of H+ secretion have more impact
pumps on the luminal membranes. For example, insect midgut
on pancreatic juice composition at low flow rates and minor
and marine fish intestine have functional V-H+-ATPase on the
at high flow rates, thus, explaining excretory curves for HCO−
luminal membranes
Also other epithelia, which are not highHCO− secretors (HCO− <25 mM), express various H+ pumps
Ca2+-ACTIVATED Cl− CHANNELS
on the luminal membranes. For example, airway epithelia trans-
In addition to CFTR-dependent secretion, a number of studies
port both base and acid, and the airway fluid layer is slightly
showed that agonists acting via Ca2+-signaling stimulate Ca2+-
acidic Some studies provide
activated Cl− channels (CaCC) and thus, could support duct
evidence for the presence of bafilomycin A sensitive V-H+ pump
secretion Winpenny
); other studies show that transport is sensitive to SCH28080,
The molecular identity of CaCC channels has been difficult to
an inhibitor of gastric (and also non-gastric) H+-K+ pumps
pinpoint [see After suggestions of CCl-2 and
bestrophins, the TMEM16/ANO family was discovered (Caputo
gastric, ouabain-sensitive H+-K+-pumps were also demonstrated
et al., ), and especially
in some studies Shan
TMEM16A/ANO1 became a CaCC favorite. Recent studies show
that human duct cell lines express TMEM16A, which re-localizes
Coming back to the pancreatic luminal H+-K+ pumps, let
from cytosol to the luminal membrane upon purinergic stimula-
us speculate what their function may be. They could help to
tion and gives rise to secretory potentials
defend the cell against intracellular acidification, although there
In human pancreatic samples immunohis-
is a redundancy of acid/base transporters including several NHEs,
tochemistry shows TMEM16A in centro-acinar and small ducts
NBCs, and Cl−/HCO− exchangers Our proposal
Novak et al.
Acid-base transport in pancreas
It is relevant to ask whether TMEM16A and/or Ca2+ signal-
ing pathways lead to HCO− secretion. There are a few studies in
Pancreatic secretion regulated by hormonal and neural systems is
support of this notion. For example, Ca2+ signaling via IRBIT
stimulates NBCe1 A
Paracrine regulation is less explored, but it is highly relevant as
recent study on TMEM16A anion permeability shows that in
it allows regulation within the gland and integration of acinar
HEK293 cell expression system and mouse salivary acinar cells
and ductal responses. Pancreatic ducts can be regulated by acinar
the channel is directly modulated by calmodulin, which increases
factors (trypsin, guanylin, ATP) as well as retrograde factors (bile
its HCO− permeability This is supported by
a study on ex vivo salivary glands stimulated with acetycholine,
which induced production of HCO− rich pancreatic-like secre-
centrate on purinergic signaling and present evidence that this
tion when Cl− transport was inhibited
signaling could fine-tune and coordinate pancreatic secretion on
Nevertheless, it cannot be excluded that there are other molec-
several fronts. Pancreatic ducts express several types of purinergic
ular candidates for CaCC, or that CFTR can convey part of the
receptors including members from the G-protein coupled recep-
Ca2+-activated Cl− currents. The latter mechanism could involve
tor families (adenosine, P2Y) and ligand-gated ion channels (P2X
Ca2+ sensitive adenylate cyclases and tyrosine kinases (Src2/Pyk
receptor) families that can potentially stimu-
complex), both of which could alter activity of CFTR, as shown
late a variety of intracellular signaling pathways
for other epithelia
Another effect at the CFTR level could be priming of some PKC
). These receptors regulate pancreatic
isoforms that enhance CFTR activity [see
duct ion transport, mucin secretion, and survival of fibrogenic
)]. Lastly, it is highly unlikely that Ca2+ mediated signaling
pancreatic stellate cells
stands alone, rather the two major signaling pathways of Ca2+ and
ATP originates from ZG where it is accumulated by the vesic-
cAMP/PKA act synergistically in pancreatic ducts, e.g., via IRBIT
ular nucleotide transporter VNUT
regulation of CFTR and SLC26A6 ).
and in addition ATP is presumably released by nerves and ductalepithelium Burnstock
K+ CHANNELS
and Novak, Various ecto-nucleotidases are expressed and
The driving force for Cl− or HCO− exit is maintained by hyper-
secreted, and potentially ATP/ADP and adenosine are effective
polarizing membrane potential created by opening of K+ chan-
regulators of ductal functions
nels, and GK is both present on the basolateral and luminal
ATP and UTP via P2 receptors have effects on intracellular
analysis has shown that stimulation of luminal K+ channels con-
Ca2+, intracellular pH, and transepithelial transport in both iso-
tributes with at least with 10% to the total conductance. Modeling
lated ducts and in vivo pancreas
in salivary glands confirms that such ratio of luminal to basolat-
). The physiological response to nucleotides is side specific.
eral K+ channels would optimize secretion without destroying the
Basolateral UTP inhibits secretion, most likely due to inhibi-
transepithelial potential and transport
tion of KCa1.1 channels, presumably to prevent overextension
Furthermore, luminal K+ channels could
of ducts. In contrast, luminal UTP/ATP application causes duct
contribute to secreted K+, as pancreatic juice contains 4–8 mM
secretion and activation and Cl− and K+ channels
). In particular KCa3.1
). The molecular identity of some K+ channels in pancre-
channel activation potentiates secretion (see above). It is well doc-
atic ducts is known, however, the exact localization and function
umented that purinergic receptor stimulation activates CFTR,
remains to be verified [see )]. The
Cl−/HCO− exchangers and TMEM16A on the luminal mem-
KCa1.1 channels (maxi-K, BK, coded by KCNMA1) are present
brane Furthermore,
in pancreatic ducts ).
P2 receptors activate CaCC and CFTR interdependently and syn-
The latter study proposes that these channels are expressed on the
ergistically, though exact receptors and signaling pathways remain
luminal membrane and activated by low concentrations of bile
to be elucidated (see above). In addition, some effects can be due
acids. However, earlier patch-clamp studies indicated that these
to stimulation of A2A and A2B receptors, which stimulate CFTR
channels were also located basolaterally Hede
et al., The KCa3.1 channel (IK, SK4, coded by KCNN4)
A number of processes in purinergic signaling are pH sen-
was demonstrated in pancreatic ducts Jung
sitive, and this must be of relevance in pancreatic duct lumen.
et al., Immunolocalization indicates
For example, nucleotidase activities, CD39 and CD73 types, are
that KCa3.1 is expressed on both membranes, though stronger on
stimulated at alkaline pH 8–9
the luminal one Interestingly, the channel activator
), thus, favoring conversion of ATP to adenosine in duct
EBIO enhanced secretion potentials Wang
lumen. Furthermore, purinergic receptors are also pH sensitive.
et al., ). Recent studies on pancreatic ducts offers molecu-
From other preparations we know that extracellular acidifica-
lar identities of several K+ channels, including KVLQT1, HERG,
tion enhanced the potency of UTP up to 10 fold on the rat
EAG2; Slick, and Slack and interestingly the
P2Y4 but not P2Y2 receptors ), and the
pH sensor TASK-2 However, the function and
P2X2 receptors was activated by acid pH
regulation of these channels in pancreas physiology needs to be
Extracellular alkalinization enhances activity the P2X4 and P2X7
receptors Several types
Novak et al.
Acid-base transport in pancreas
of these receptors are expressed in duct lumen including the
Bilbao, P. S., Katz, S., and Boland, R. (2012). Interaction of purinergic recep-
P2Y2 and P2X7 receptors, and these enhance pancreatic secretion
tors with GPCRs, ion channels, tyrosine kinase and steroid hormone receptors
and integrate acini-to-duct signaling
orchestrates cell function. Purinergic Signal. 8, 91–103. doi: 10.1007/s11302-011-9260-9
Billet, A., and Hanrahan, J. W. (2013). The secret life of CFTR as a calcium-activated
chloride channel. J. Physiol. 591, 5273–5278. doi: 10.1113/jphysiol.2013.261909
SUMMARY AND PERSPECTIVES
Billet, A., Luo, Y., Balghi, H., and Hanrahan, J. W. (2013). Role of tyrosine phos-
The original cellular model for pancreatic HCO− secretion has
phorylation in the muscarinic activation of the Cystic Fibrosis Transmembrane
Conductance Regulator (CFTR). J. Biol. Chem. 288, 21815–21823. doi:
been supplemented with molecular identities for many ion trans-
porters/channels. The present review challenges present concepts
Bodin, P., and Burnstock, G. (2001). Purinergic signaling: ATP release. Neurochem.
by including active H+ pumps in the model, and by compar-
Res. 26, 959–969. doi: 10.1023/A:1012388618693
ing basic processes in pancreas and stomach. Furthermore, we
Bouwens, L., and Pipeleers, D. G. (1998). Extra-insular beta cells associated with
present new additions to the model—Ca2+-activated Cl− and K+
ductules are frequent in adult human pancreas. Diabetologia 41, 629–633. doi:10.1007/s001250050960
channels, and propose that they work in synergy to regulate secre-
Bro-Rasmussen, F., Killmann, S. A., and Thaysen, J. H. (1956). The composition
tion. On the organ level, acini, and ducts integrate their function
of pancreatic juice as compared to sweat, parotid saliva and tears. Acta Physiol.
in acid/base transport and regulation, the latter exemplified by
Scand. 37, 97–113. doi: 10.1111/j.1748-1716.1956.tb01346.x
purinergic signaling. Further challenges lay in understanding dys-
Brown, D., and Wagner, C. A. (2012). Molecular mechanisms of acid-base sensing
by the kidney. J. Am. Soc. Nephrol. 23, 774–780. doi: 10.1681/ASN.2012010029
regulation of acid-base transport in pancreas pathophysiology. In
Burnstock, G. (2007). Purine and pyrimidine receptors. Cell Mol. Life Sci. 64,
CF patients and animal models, pancreatic juice pH decreases
1471–1483. doi: 10.1007/s00018-007-6497-0
from values >8.1 to <6.6, and pancreas contributes to duode-
Burnstock, G., and Novak, I. (2012). Purinergic signaling in the pancreas in health
nal hyperacidity [see
and disease. J. Endocrinol. 213, 123–141. doi: 10.1530/JOE-11-0434
It is not clear whether the prob-
Caflisch, C. R., Solomon, S., and Galey, W. R. (1979). Exocrine ductal pCO2 in the
rabbit pancreas. Pflugers Arch. 380, 121–125. doi: 10.1007/BF00582146
lem relates to ductal and/or acinar secretion. In acute pancreatitis,
Caputo, A., Caci, E., Ferrera, L., Pedemonte, N., Barsanti, C., Sondo, E.,
which has complex etiologies, it is now appreciated that defec-
et al. (2008). TMEM16A, a membrane protein associated with calcium-
tive pancreatic duct secretion can be the initiating factor (Lee
dependent chloride channel activity. Science 322, 590–594. doi: 10.1126/science.
and Muallem, Finally, in several cancer
types, various acid-base transporters and associated ion channels,
Case, R. M., and Argent, B. E. (1993). "Pancreatic duct cell secretion: control and
mechanims of transport," in The Pancreas. Biology, Pathobiology, and Diseases,
such as NHE1, NBCn1, CAIX, TMEM16A, Kv10.1, and KCa3.1,
eds V. L. W. Go, E. P. DiMagno, J. D. Gardner, E. Lebenthal, H. A. Reber, and G.
change expression or function [see )]. Our
A. Scheele (New York, NY: Raven Press), 301–350.
knowledge about the role of acid-base transporters in pancreatic
Case, R. M., Harper, A. A., and Scratcherd, T. (1969). The secretion of electrolytes
ductal adenocarcinoma clearly needs to be expanded, in order to
and enzymes by the pancreas of the anaesthetized cat. J. Physiol. (Lond.) 201,
provide potential diagnostic and therapeutic approaches.
Chu, S., and Schubert, M. L. (2012). Gastric secretion. Curr. Opin. Gastroenterol.
28, 587–593. doi: 10.1097/MOG.0b013e328358e5cc
Clarke, C. E., Benham, C. D., Bridges, A., George, A. R., and Meadows, H. J.
Research projects founding basis for this review were supported
(2000). Mutation of histidine 286 of the human P2X4 purinoceptor removes
by The Danish Council for Independent Research Natural
extracellular pH sensitivity. J. Physiol 523 (pt 3), 697–703. doi: 10.1111/j.1469-7793.2000.00697.x
Sciences, The Lundbeck Foundation, The Novo Nordisk
Coakley, R. D., Grubb, B. R., Paradiso, A. M., Gatzy, J. T., Johnson, L. G., Kreda,
Foundation and The Carlsberg Foundation.
S. M., et al. (2003). Abnormal surface liquid pH regulation by cultured cysticfibrosis bronchial epithelium. Proc. Natl. Acad. Sci. U.S.A. 100, 16083–16088.
doi: 10.1073/pnas.2634339100
Cook, D. I., and Young, J. A. (1989). Effect of K+ channels in the apical plasma
Almassy, J., Won, J. H., Begenisich, T. B., and Yule, D. I. (2012). Apical Ca2+-
membrane on epithelial secretion based on secondary active Cl− transport.
activated potassium channels in mouse parotid acinar cells. J. Gen. Physiol. 139,
J. Membr. Biol. 110, 139–146. doi: 10.1007/BF01869469
121–133. doi: 10.1085/jgp.201110718
DeCoursey, T. E. (2013). Voltage-gated proton channels: molecular biology, phys-
Alvarez, C., Regan, J. P., Merianos, D., and Bass, B. L. (2004). Protease-activated
iology, and pathophysiology of the H(V) family. Physiol. Rev. 93, 599–652. doi:
receptor-2 regulates bicarbonate secretion by pancreatic duct cells in vitro.
Surgery 136, 669–676. doi: 10.1016/j.surg.2004.01.018
de Ondarza, J., and Hootman, S. R. (1997). Confocal microscopic analysis of intra-
Ashizawa, N., Endoh, H., Hidaka, K., Watanabe, M., and Fukumoto, S. (1997).
cellular pH regulation in isolated guinea pig pancreatic ducts. Am. J. Physiol.
Three-dimensional structure of the rat pancreatic duct in normal and inflam-
272, G124–G134.
mated pancreas. Microsc. Res. Tech. 37, 543–556. doi: 10.1002/(SICI)1097-
Duran, C., Thompson, C. H., Xiao, Q., and Hartzell, H. C. (2010). Chloride chan-
nels: often enigmatic, rarely predictable. Annu. Rev. Physiol. 72, 95–121. doi:
Ashley, S. W., Schwarz, M., Alvarez, C., Nguyen, T. N., Vdovenko, A., and Reber, H.
A. (1994). Pancreatic interstitial pH regulation: effects of secretory stimulation.
Fernandez-Salazar, M. P., Pascua, P., Calvo, J. J., Lopez, M. A., Case, R. M., Steward,
Surgery 115, 503–509.
M. C., et al. (2004). Basolateral anion transport mechanisms underlying fluid
Behrendorff, N., Floetenmeyer, M., Schwiening, C., and Thorn, P. (2010). Protons
secretion by mouse, rat and guinea-pig pancreatic ducts. J. Physiol. (Lond.) 556,
released during pancreatic acinar cell secretion acidify the lumen and con-
415–428. doi: 10.1113/jphysiol.2004.061762
tribute to pancreatitis in mice. Gastroenterology 139, 1711-20, 1720.e1-5. doi:
Fischer, H., and Widdicombe, J. H. (2006). Mechanisms of acid and base secretion
by the airway epithelium. J. Membr. Biol. 211, 139–150. doi: 10.1007/s00232-
Bergmann, F., Andrulis, M., Hartwig, W., Penzel, R., Gaida, M. M., Herpel,
E., et al. (2011). Discovered on gastrointestinal stromal tumor 1 (DOG1)
Fong, P., Argent, B. E., Guggino, W. B., and Gray, M. A. (2003). Characterization
is expressed in pancreatic centroacinar cells and in solid-pseudopapillary
of vectorial chloride transport pathways in the human pancreatic duct adeno-
neoplasms–novel evidence for a histogenetic relationship. Hum. Pathol. 42,
carcinoma cell line, HPAF. Am. J. Physiol. Cell Physiol. 285, C433–C445. doi:
817–823. doi: 10.1016/j.humpath.2010.10.005
Novak et al.
Acid-base transport in pancreas
Forte, J. G., and Zhu, L. (2010). Apical recycling of the gastric parietal cell H,K-
(NTPDase1) and NTPDase2 in Pancreas and Salivary Gland. J. Histochem.
ATPase. Annu. Rev. Physiol. 72, 273–296. doi: 10.1146/annurev-physiol-021909-
Cytochem. 52, 861–871. doi: 10.1369/jhc.3A6167.2004
Kodama, T. (1983). A light and electron microscopic study on the pancreatic ductal
Freedman, S. D., Kern, H. F., and Scheele, G. A. (2001). Pancreatic acinar cell dys-
system. Acta Pathol. Jpn. 33, 297–321.
function in CFTR(-/-) mice is associated with impairments in luminal pH and
Krouse, M. E., Talbott, J. F., Lee, M. M., Joo, N. S., and Wine, J. J. (2004). Acid and
endocytosis. Gastroenterology 121, 950–957. doi: 10.1053/gast.2001.27992
base secretion in the Calu-3 model of human serous cells. Am. J. Physiol. Lung.
Gray, M. A., Greenwell, J. R., Garton, A. J., and Argent, B. E. (1990). Regulation of
Cell Mol. Physiol. 287, L1274–L1283. doi: 10.1152/ajplung.00036.2004
maxi-K+ channels on pancreatic duct cells by cyclic AMP-dependent phospho-
Kulaksiz, H., Schmid, A., Honscheid, M., Eissele, R., Klempnauer, J., and
rylation. J. Membr. Biol. 115, 203–215. doi: 10.1007/BF01868636
Cetin, Y. (2001). Guanylin in the human pancreas: a novel luminocrine
Gray, M. A., Harris, A., Coleman, L., Greenwell, J. R., and Argent, B. E. (1989). Two
regulatory pathway of electrolyte secretion via cGMP and CFTR in
types of chloride channel on duct cells cultured from human fetal pancreas. Am.
the ductal system. Histochem. Cell Biol. 115, 131–145. doi: 10.1007/
J. Physiol. 257, C240–C251.
Grotmol, T., Buanes, T., Bros, O., and Raeder, M. G. (1986). Lack of effect of
Leal, D. B., Streher, C. A., Neu, T. N., Bittencourt, F. P., Leal, C. A., da Silva,
amiloride, furosemide, bumetanide and triamterene on pancreatic NaHCO3
J. E., et al. (2005). Characterization of NTPDase (NTPDase1; ecto-apyrase;
secretion in pigs. Acta Physiol. Scand. 126, 593–600. doi: 10.1111/j.1748-
ecto-diphosphohydrolase; CD39; EC 3.6.1.5) activity in human lymphocytes.
Biochim. Biophys. Acta 1721, 9–15. doi: 10.1016/j.bbagen.2004.09.006
Guffey, S., Esbaugh, A., and Grosell, M. (2011). Regulation of apical H+-
Lee, M. G., and Muallem, S. (2008). Pancreatitis: the neglected duct. Gut 57,
ATPase activity and intestinal HCO− secretion in marine fish osmoregula-
1037–1039. doi: 10.1136/gut.2008.150961
tion. Am. J. Physiol. Regul. Integr. Comp. Physiol. 301, R1682–R1691. doi:
Lee, M. G., Ohana, E., Park, H. W., Yang, D., and Muallem, S. (2012). Molecular
mechanism of pancreatic and salivary gland fluid and HCO− secretion. Physiol.
Haanes, K. A., and Novak, I. (2010). ATP storage and uptake by isolated pancreatic
Rev. 92, 39–74. doi: 10.1152/physrev.00011.2011
zymogen granules. Biochem. J. 429, 303–311. doi: 10.1042/BJ20091337
Lenertz, L. Y., Gavala, M. L., Zhu, Y., and Bertics, P. J. (2011). Transcriptional
Haanes, K. A., Schwab, A., and Novak, I. (2012). The P2X7 receptor supports both
control mechanisms associated with the nucleotide receptor P2X7, a critical reg-
life and death in fibrogenic pancreatic stellate cells. PLoS ONE 7:e51164. doi:
ulator of immunologic, osteogenic, and neurologic functions. Immunol. Res. 50,
22–38. doi: 10.1007/s12026-011-8203-4
Hayashi, M., and Novak, I. (2013). Molecular basis of potassium channels in
Liu, X., Ma, W., Surprenant, A., and Jiang, L. H. (2009). Identification of the
pancreatic duct epithelial cells. Channels (Austin) 7, 1–10. doi: 10.4161/chan.
amino acid residues in the extracellular domain of rat P2X(7) receptor involved
in functional inhibition by acidic pH. Br. J. Pharmacol. 156, 135–142. doi:
Hayashi, M., Wang, J., Hede, S. E., and Novak, I. (2012). An intermediate-
conductance Ca2+-activated K+ channel is important for secretion in pancre-
Namkung, W., Lee, J. A., Ahn, W., Han, W., Kwon, S. W., Ahn, D. S., et al. (2003).
atic duct cells. Am. J. Physiol. Cell Physiol. 303, C151–C159. doi: 10.1152/ajp-
Ca2+ activates cystic fibrosis transmembrane conductance regulator- and Cl− -
dependent HCO3 transport in pancreatic duct cells. J. Biol. Chem. 278, 200–207.
Hede, S. E., Amstrup, J., Christoffersen, B. C., and Novak, I. (1999). Purinoceptors
evoke different electrophysiological responses in pancreatic ducts. P2Y inhibits
Niv, Y., and Fraser, G. M. (2002). The alkaline tide phenomenon. J. Clin.
K+ conductance, and P2X stimulates cation conductance. J. Biol. Chem. 274,
Gastroenterol. 35, 5–8. doi: 10.1097/00004836-200207000-00003
31784–31791. doi: 10.1074/jbc.274.45.31784
Novak, I. (2008). Purinergic receptors in the endocrine and exocrine pancreas.
Hede, S. E., Amstrup, J., Klaerke, D. A., and Novak, I. (2005). P2Y2 and P2Y4 recep-
Purinergic Signal. 4, 237–253. doi: 10.1007/s11302-007-9087-6
tors regulate pancreatic Ca2+-activated K+ channels differently. Pflugers Arch.
Novak, I. (2011). Purinergic signaling in epithelial ion transport—regulation of
450, 429–436. doi: 10.1007/s00424-005-1433-3
secretion and absorption. Acta Physiologica 202, 501–522. doi: 10.1111/j.1748-
Hegyi, P., Maleth, J., Venglovecz, V., and Rakonczay, Z. Jr. (2011a). Pancreatic ductal
bicarbonate secretion: challenge of the acinar Acid load. Front. Physiol. 2:36. doi:
Novak, I., and Greger, R. (1988). Electrophysiological study of transport systems
in isolated perfused pancreatic ducts: properties of the basolateral membrane.
Hegyi, P., Pandol, S., Venglovecz, V., and Rakonczay, Z. Jr. (2011b). The acinar-
Pflügers Arch. 411, 58–68. doi: 10.1007/BF00581647
ductal tango in the pathogenesis of acute pancreatitis. Gut 60, 544–552. doi:
Novak, I., and Greger, R. (1991). Effect of bicarbonate on potassium conduc-
tance of isolated perfused rat pancreatic ducts. Pflügers Arch. 419, 76–83. doi:
Inglis, S. K., Wilson, S. M., and Olver, R. E. (2003). Secretion of acid and base
equivalents by intact distal airways. Am. J. Physiol. Lung. Cell Mol. Physiol. 284,
Novak, I., Hede, S. E., and Hansen, M. R. (2008). Adenosine receptors in rat
L855–L862. doi: 10.1152/ajplung.00348.2002
and human pancreatic ducts stimulate chloride transport. Pflugers Arch. 456,
Ishiguro, H., Naruse, S., Kitagawa, M., Hayakawa, T., Case, R. M., and Steward,
437–447. doi: 10.1007/s00424-007-0403-3
M. C. (1999). Luminal ATP stimulates fluid and HCO− secretion in guinea-
Novak, I., Jans, I. M., and Wohlfahrt, L. (2010). Effect of P2X7 receptor knockout
pig pancreatic duct. J. Physiol. (Lond) 519, 551–558. doi: 10.1111/j.1469-
on exocrine secretion of pancreas, salivary glands and lacrimal glands. J. Physiol.
(Lond) 588(pt 18), 3615–3627. doi: 10.1113/jphysiol.2010.190017
Ishiguro, H., Steward, M. C., Wilson, R. W., and Case, R. M. (1996). Bicarbonate
Novak, I., Wang, J., Henriksen, K. L., Haanes, K. A., Krabbe, S., Nitschke, R., et al.
secretion in interlobular ducts from guinea-pig pancreas. J. Physiol. (Lond.) 495
(2011). Pancreatic bicarbonate secretion involves two proton pumps. J. Biol.
(pt 1), 179–191.
Chem. 286, 280–289. doi: 10.1074/jbc.M110.136382
Jung, J., Nam, J. H., Park, H. W., Oh, U., Yoon, J. H., and Lee, M. G. (2013).
Novak, I., and Young, J. A. (1986). Two independent anion transport sys-
Dynamic modulation of ANO1/TMEM16A. Proc. Natl. Acad. Sci. U.S.A. 110,
tems in rabbit mandibular salivary glands. Pflugers Arch. 407, 649–656. doi:
360–365. doi: 10.1073/pnas.1211594110
Jung, S. R., Kim, K., Hille, B., Nguyen, T. D., and Koh, D. S. (2006). Pattern of Ca2+
Pahl, C., and Novak, I. (1993). Effect of vasoactive intestinal peptide, carbachol and
increase determines the type of secretory mechanism activated in dog pancreatic
other agonists on cell membrane voltage of pancreatic duct cells. Pflügers Arch.
duct epithelial cells. J. Physiol. 576, 163–178. doi: 10.1113/jphysiol.2006.114876
424, 315–320. doi: 10.1007/BF00384358
Jung, S. R., Kim, M. H., Hille, B., Nguyen, T. D., and Koh, D. S. (2004). Regulation
Pallagi, P., Venglovecz, V., Rakonczay, Z. Jr., Borka, K., Korompay, A., Ozsvari, B.,
of exocytosis by purinergic receptors in pancreatic duct epithelial cells. Am. J.
et al. (2011). Trypsin reduces pancreatic ductal bicarbonate secretion by inhibit-
Physiol. Cell Physiol. 286, C573–C579. doi: 10.1152/ajpcell.00350.2003
ing CFTR Cl channels and luminal anion exchangers. Gastroenterology 141,
King, B. F., Ziganshina, L. E., Pintor, J., and Burnstock, G. (1996). Full sensitiv-
2228–2239. doi: 10.1053/j.gastro.2011.08.039
ity of P2X2 purinoceptor to ATP revealed by changing extracellular pH. Br. J.
Park, S., Shcheynikov, N., Hong, J. H., Zheng, C., Suh, S. H., Kawaai, K.,
Pharmacol. 117, 1371–1373. doi: 10.1111/j.1476-5381.1996.tb15293.x
et al. (2013). Irbit mediates synergy between Ca2+ and cAMP signaling path-
Kittel, A., Pelletier, J., Bigonnesse, F., Guckelberger, O., Kordas, K., Braun, N.,
ways during epithelial transport in mice. Gastroenterology 145, 232–241. doi:
et al. (2004). Localization of Nucleoside Triphosphate Diphosphohydrolase-1
Novak et al.
Acid-base transport in pancreas
Pascua, P., Garcia, M., Fernandez-Salazar, M. P., Hernandez-Lorenzo, M. P., Calvo,
Ca2+-activated potassium channels in pancreatic duct epithelial cells. Gut 60,
J. J., Colledge, W. H., et al. (2009). Ducts isolated from the pancreas of CFTR-
361–369. doi: 10.1136/gut.2010.214213
null mice secrete fluid. Pflugers Arch. 459, 203–214. doi: 10.1007/s00424-009-
Venglovecz, V., Rakonczay, Z. Jr., Ozsvari, B., Takacs, T., Lonovics, J., Varro, A., et al.
(2008). Effects of bile acids on pancreatic ductal bicarbonate secretion in guinea
Pedersen, S. F., Hoffmann, E. K., and Novak, I. (2013). Cell volume reg-
pig. Gut 57, 1102–1112. doi: 10.1136/gut.2007.134361
ulation in epithelial physiology and cancer. Front. Physiol. 4, 233. doi:
Villanger, O., Veel, T., and Raeder, M. G. (1995). Secretin causes H+/HCO−
secretion from pig pancreatic ductules by vacuolar-type H+-adenosine triphos-
Poulsen, J. H., and Machen, T. E. (1996). HCO3-dependent pHi regulation in tra-
phatase. Gastroenterology 108, 850–859. doi: 10.1016/0016-5085(95)90460-3
cheal epithelial cells. Pflugers Arch. 432, 546–554. doi: 10.1007/s004240050168
Wang, J., Haanes, K. A., and Novak, I. (2013). Purinergic regulation of CFTR
Roussa, E., Alper, S. L., and Thevenod, F. (2001). Immunolocalization of
and Ca2+-activated Cl− channels and K+ channels in human pancreatic duct
anion exchanger AE2, Na+/H+ exchangers NHE1 and NHE4, and vacuolar
epithelium. Am. J. Physiol. Cell Physiol. 304, C673–C684. doi: 10.1152/ajp-
type H+-ATPase in rat pancreas. J. Histochem. Cytochem. 49, 463–474. doi:
Wang, J., and Novak, I. (2013). Ion transport in human pancreatic duct epithe-
Rucker, B., Almeida, M. E., Libermann, T. A., Zerbini, L. F., Wink, M. R., and Sarkis,
lium, Capan-1 cells, is regulated by secretin, VIP, acetylcholine, and purinergic
J. J. (2008). E-NTPDases and ecto-5'-nucleotidase expression profile in rat heart
receptors. Pancreas 42, 452–460. doi: 10.1097/MPA.0b013e318264c302
left ventricle and the extracellular nucleotide hydrolysis by their nerve terminal
Wang, T., Busk, M., and Overgaard, J. (2001). The respiratory consequences of feed-
endings. Life Sci. 82, 477–486. doi: 10.1016/j.lfs.2007.12.003
ing in amphibians and reptiles. Comp. Biochem. Physiol. A Mol. Integr. Physiol.
Rune, S. J., and Lassen, N. A. (1968). Diurnal variations in the acid-base balance of
128, 535–549. doi: 10.1016/S1095-6433(00)00334-2
blood. Scand. J. Clin. Lab. Invest. 22, 151–156. doi: 10.3109/00365516809160961
Wieczorek, H., Beyenbach, K. W., Huss, M., and Vitavska, O. (2009). Vacuolar-
Sachs, G., Shin, J. M., and Hunt, R. (2010). Novel approaches to inhibition of gastric
type proton pumps in insect epithelia. J. Exp. Biol. 212, 1611–1619. doi:
acid secretion. Curr. Gastroenterol. Rep. 12, 437–447. doi: 10.1007/s11894-010-
Wildman, S. S., Unwin, R. J., and King, B. F. (2003). Extended pharmacological pro-
Sachs, G., Shin, J. M., Vagin, O., Lambrecht, N., Yakubov, I., and Munson, K.
files of rat P2Y2 and rat P2Y4 receptors and their sensitivity to extracellular H+
(2007). The gastric H,K ATPase as a drug target: past, present, and future. J. Clin.
and Zn2+ ions. Br. J. Pharmacol. 140, 1177–1186. doi: 10.1038/sj.bjp.0705544
Gastroenterol. 41 (Suppl. 2), S226–S242. doi: 10.1097/MCG.0b013e31803233b7
Wiley, J. S., Sluyter, R., Gu, B. J., Stokes, L., and Fuller, S. J. (2011). The human
Schroeder, B. C., Cheng, T., Jan, Y. N., and Jan, L. Y. (2008). Expression cloning
P2X7 receptor and its role in innate immunity. Tissue Antigens 78, 321–332. doi:
of TMEM16A as a calcium-activated chloride channel subunit. Cell 134,
1019–1029. doi: 10.1016/j.cell.2008.09.003
Wilschanski, M., and Novak, I. (2013). The cystic fibrosis of exocrine pancreas. Cold
Seow, K. T. F. P., Case, R. M., and Young, J. A. (1991). Pancreatic secretion by
Spring Harb. Perspect. Med. 3, a009746. doi: 10.1101/cshperspect.a009746
the anaesthetized rabbit in response to secretin, cholecystokinin, and carbachol.
Winpenny, J. P., Harris, A., Hollingsworth, M. A., Argent, B. E., and Gray, M.
Pancreas 6, 385–391. doi: 10.1097/00006676-199107000-00002
A. (1998). Calcium-activated chloride conductance in a pancreatic adenocarci-
Sewell, W. A., and Young, J. A. (1975). Secretion of electrolytes by the pancreas of
noma cell line of ductal origin (HPAF) and in freshly isolated human pancreatic
the anaesthetized rat. J. Physiol. (Lond.) 252, 379–396.
duct cells. Pflugers Arch. 435, 796–803. doi: 10.1007/s004240050586
Shan, J., Liao, J., Huang, J., Robert, R., Palmer, M. L., Fahrenkrug, S. C., et al.
Wood, C. M., Bucking, C., and Grosell, M. (2010). Acid-base responses to feed-
(2012). Bicarbonate-dependent chloride transport drives fluid secretion by
ing and intestinal Cl− uptake in freshwater- and seawater-acclimated killi-
the human airway epithelial cell line Calu-3. J. Physiol. 590, 5273–5297. doi:
fish, Fundulus heteroclitus, an agastric euryhaline teleost. J. Exp. Biol. 213,
2681–2692. doi: 10.1242/jeb.039164
Shirakabe, K., Priori, G., Yamada, H., Ando, H., Horita, S., Fujita, T., et al. (2006).
Yang, D., Shcheynikov, N., Zeng, W., Ohana, E., So, I., Ando, H., et al. (2009). IRBIT
IRBIT, an inositol 1,4,5-trisphosphate receptor-binding protein, specifically
coordinates epithelial fluid and HCO− secretion by stimulating the transporters
binds to and activates pancreas-type Na+/HCO− cotransporter 1 (pNBC1).
pNBC1 and CFTR in the murine pancreatic duct. J. Clin. Invest. 119, 193–202.
Proc. Natl. Acad. Sci. U.S.A. 103, 9542–9547. doi: 10.1073/pnas.0602250103
doi: 10.1172/JCI36983
Smith, J. J., and Welsh, M. J. (1993). Fluid and electrolyte transport by cultured
Yang, Y. D., Cho, H., Koo, J. Y., Tak, M. H., Cho, Y., Shim, W. S., et al. (2008).
human airway epithelia. J. Clin. Invest. 91, 1590–1597. doi: 10.1172/JCI116365
TMEM16A confers receptor-activated calcium-dependent chloride conduc-
Sørensen, C. E., Amstrup, J., Rasmussen, H. N., Ankorina-Stark, I., and Novak,
tance. Nature 455, 1210–1215. doi: 10.1038/nature07313
I. (2003). Rat pancreas secretes particulate ecto-nucleotidase CD39. J. Physiol.
Yegutkin, G. G., Samburski, S. S., Jalkalen, S., and Novak, I. (2006). ATP-
(Lond.) 551, 881–892. doi: 10.1113/jphysiol.2003.049411
consuming and ATP-generating enzymes secreted by pancreas. J. Biol. Chem.
Sørensen, C. E., and Novak, I. (2001). Visualization of ATP release in pancreatic
281, 29441–29447. doi: 10.1074/jbc.M602480200
acini in response to cholinergic stimulus. Use of fluorescent probes and confocal
You, C. H., Rominger, J. M., and Chey, W. Y. (1983). Potentiation effect of
microscopy. J. Biol. Chem. 276, 32925–32932. doi: 10.1074/jbc.M103313200
cholecystokinin-octapeptide on pancreatic bicarbonate secretion stimulated by
Steward, M. C., and Ishiguro, H. (2009). Molecular and cellular regulation of
a physiologic dose of secretin in humans. Gastroenterology 85, 40–45.
pancreatic duct cell function. Curr. Opin. Gastroenterol. 25, 447–453. doi:
Zhao, H., Star, R. A., and Muallem, S. (1994). Membrane localization of H+ and
HCO− transporters in the rat pancreatic ducts. J. Gen. Physiol. 104, 57–85. doi:
Steward, M. C., Ishiguro, H., and Case, R. M. (2005). Mechanisms of bicarbon-
ate secretion in the pancreatic duct. Annu. Rev. Physiol. 67, 377–409. doi:10.1146/annurev.physiol.67.031103.153247
Conflict of Interest Statement: The authors declare that the research was con-
Surprenant, A., and North, R. A. (2009). Signaling at purinergic P2X recep-
ducted in the absence of any commercial or financial relationships that could be
tors. Annu. Rev. Physiol. 71, 333–359. doi: 10.1146/annurev.physiol.70.113006.
construed as a potential conflict of interest.
Szalmay, G., Varga, G., Kajiyama, F., Yang, X. S., Lang, T. F., Case, R. M., et al.
Received: 07 October 2013; paper pending published: 23 October 2013; accepted: 04
(2001). Bicarbonate and fluid secretion evoked by cholecystokinin, bombesin
December 2013; published online: 20 December 2013.
and acetylcholine in isolated guinea-pig pancreatic ducts. J. Physiol. (Lond.) 535,
Citation: Novak I, Haanes KA and Wang J (2013) Acid-base transport in pancreas—
795–807. doi: 10.1111/j.1469-7793.2001.00795.x
new challenges. Front. Physiol. 4:380. doi:
Tresguerres, M., Buck, J., and Levin, L. R. (2010). Physiological carbon dioxide,
This article was submitted to Membrane Physiology and Membrane Biophysics, a
bicarbonate, and pH sensing. Pflugers Arch. 460, 953–964. doi: 10.1007/s00424-
section of the journal Frontiers in Physiology.
Copyright 2013 Novak, Haanes and Wang. This is an open-access article dis-
Uc, A., Stoltz, D. A., Ludwig, P., Pezzulo, A., Griffin, M., bu-El-Haija, M., et al.
tributed under the terms of the The
(2011). Pancreatic and biliary secretion differ in cystic fibrosis and wild-type
use, distribution or reproduction in other forums is permitted, provided the original
pigs. J. Cystic Fibrosis 10, S69. doi: 10.1016/S1569-1993(11)60285-3
author(s) or licensor are credited and that the original publication in this jour-
Venglovecz, V., Hegyi, P., Rakonczay, Z. Jr., Tiszlavicz, L., Nardi, A., Grunnet,
nal is cited, in accordance with accepted academic practice. No use, distribution or
M., et al. (2011). Pathophysiological relevance of apical large-conductance
reproduction is permitted which does not comply with these terms.
Source: http://curis.ku.dk/ws/files/99246236/Acid_basetransportinpancreas_newchallenges.pdf
Untitled
Age and Ageing 2015; 44: 213–218 © The Author 2014. Published by Oxford University Press on behalf of the British Geriatrics Society. This is an Open Access article distributed under the terms of the Creative Commons Attribution Published electronically 16 October 2014 Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0/), which permits non-commercial re-use, distribution, and reproduction in any medium, provided the original work is
Microsoft word - 2014-202726-1 summary brochure-v
2014–2015 Student Injury and Sickness Insurance Plan for College of Coastal Georgia Who is eligible to enroll? All students enrolled for six (6) or more credits per term or participating in Cooperative Education Programs are eligible to enroll in the insurance plan, excluding those students that are required to enroll in the plan as stated below. Eligible Dependents of enrolled students may participate in the plan on a voluntary basis. The students required to enroll in the plan are as follows: 1. All undergraduate and ESL international students holding F or J visas. 2. All undergraduate students enrolled in programs that require proof of health insurance. 3. All graduate students receiving a full tuition waiver as part of their graduate assistantship award. 4. All graduate international students and visiting scholars holding F or J visas. 5. All graduate students enrolled in programs that require proof of health insurance. 6. All graduate students receiving fellowships that fully fund their tuition. Eligible Dependents are the student's legal spouse and dependent children under 26 years of age. Where can I get more information about the benefits available? Please read the plan brochure to determine whether this plan is right before you enroll. The plan brochure provides details of the coverage including costs, benefits, exclusions, and reductions or limitations and the terms under which the coverage may be continued in force. Copies of the plan brochure are available from the University and may be viewed at www.uhcsr.com/usg. Who can answer questions I have about the plan? If you have questions please contact Customer Service at 1-866-403-8267 or [email protected]. What important dates or deadlines should I be aware of? Online waivers must be submitted by September 20, 2014. How much does the plan cost?