Pnas201006899 22145.22150

Dissection of SNARE-driven membrane fusion andneuroexocytosis by wedging small hydrophobicmolecules into the SNARE zipperYoosoo Yanga,1, Jae Yoon Shina,1, Jung-Mi Oha, Chang Hwa Junga, Yunha Hwanga, Sehyun Kima, Jun-Seob Kima,Kee-Jung Yoona, Ji-Young Ryub, Jaeil Shinc, Jae Sung Hwangd, Tae-Young Yoonb, Yeon-Kyun Shinc,e,2,and Dae-Hyuk Kweona,2
aSchool of Life Science and Biotechnology and Center for Human Interface Nanotechnology, Sungkyunkwan University, Suwon 440-746, South Korea;
bDepartment of Physics, Korea Advanced Institute of Science and Technology (KAIST), Daejeon 760-749, South Korea; cDepartment of Biochemistry,Biophysics, and Molecular Biology, Iowa State University, Ames, IA 50011; dSkin Biotechnology Center, Graduate School of Biotechnology and Institute of LifeScience and Resources, Kyung Hee University, Yongin 446-701, Korea; and eIntegrative Biology and Biotechnology, Pohang University of Science andTechnology (POSTECH), Pohang 790-784, Korea
Edited by Josep Rizo, University of Texas Southwestern Medical Center, Dallas, TX, and accepted by the Editorial Board November 8, 2010 (received for reviewMay 18, 2010)
Neuronal SNARE proteins mediate neurotransmitter release at the
allowed us to understand the basic architecture of the putative
synapse by facilitating the fusion of vesicles to the presynaptic
plasma membrane. Cognate v-SNAREs and t-SNAREs from thevesicle and the plasma membrane, respectively, zip up and bring
about the apposition of two membranes attached at the C-
SNARE-Driven Membrane Fusion Can Be Controlled by SHMs with
terminal ends. Here, we demonstrate that SNARE zippering can
Different Modes of Action. As an initial step to examine the feasi-
be modulated in the midways by wedging with small hydrophobic
bility of whether SHM works as a wedge for the SNARE zippering,
molecules. Myricetin, which intercalated into the hydrophobic in-
39 polyphenolic compounds representing 12 subgroups were
ner core near the middle of the SNARE complex, stopped SNARE
screened for inhibitory activity against SNARE-driven proteoli-
zippering in motion and accumulated the trans-complex, where
posome fusion (11). We used polyphenolic compounds as a source
the N-terminal region of v-SNARE VAMP2 is in the coiled coil with
for SHM because they are abundant in nature and are known to be
the frayed C-terminal region. Delphinidin and cyanidin inhibited
versatile in helical bundle binding (12–15). The SHMs displayed
N-terminal nucleation of SNARE zippering. Neuronal SNARE com-
a wide range of inhibitory activity during the initial screen, which
plex in PC12 cells showed the same pattern of vulnerability to
was conducted at a concentration of 20 μM, equivalent to the
small hydrophobic molecules. We propose that the half-zippedtrans
concentration of t-SNARE proteins used in the fusion assay
-SNARE complex is a crucial intermediate waiting for a calcium
The nine most effective compounds were selected
trigger that leads to fusion pore opening.
for subsequent experiments, and the least effective compound,
polyphenol hemifusion neurotransmission neuron
kaempferol, was used as a negative control. Next, the degree ofinhibition of lipid mixing (Fig. 1A) and SNARE complex forma-
tion (Fig. 1B) were determined for each compound at a concen-
eurotransmitter release at the synapse, which serves as the
tration of 10 μM. The extent of SNARE complex formation was
brain's major form of cell–cell communication, requires the
assessed by exploiting the SDS-resistant property of the core
fusion of synaptic vesicles with the presynaptic plasma membrane.
complex (16). For all 10 compounds tested, the degree of SNARE
Soluble N-ethylmaleimide-sensitive factor attachment proteinreceptor (SNARE) proteins mediate this synaptic fusion event
complex formation inhibition correlated well with that of lipid-
(1–5), and the formation of a four-helical bundle (6–8) is believed
mixing inhibition (Fig. 1C), suggesting that SHM-mediated in-
to generate the force required for fusion. A zipper model has
hibition of membrane fusion is likely a direct consequence of in-
been proposed for SNARE complex formation, initiating as-
hibition of SNARE zippering. The tested compounds did not
sembly at the N-terminal region and zipping toward the C-ter-
cause liposome fusion without SNARE proteins and did not cause
minal membrane-proximal region (6–9). To account for fast
precipitation of proteoliposomes at the concentrations tested
neuroexocytosis, the SNAREs in primed readily releasable vesicles
). We exclude the possibility that SHMs inhibited anti-
have been proposed as being partially zipped in the trans-config-
SNAP-25 antibody binding to SNARE complexes (
uration bridging the two membranes.
Thus, the results of the in vitro fusion assay indicate that certain
Although the structure of the fully assembled cis-SNARE
SHMs are capable of down-regulating SNARE complex forma-
complex, which is believed to represent the postfusion state, has
tion, thereby inhibiting membrane fusion in a concentration-
been determined (10), the structure of the trans-complex is poorly
dependent manner (
understood and is purely imaginary, most likely because of itsinherently transient nature. Precisely linking the degrees ofSNARE zippering to specific stages of membrane fusion seems to
Author contributions: Y.Y., T.-Y.Y., Y.-K.S., and D.-H.K. designed research; Y.Y., J.Y.S.,
be prerequisite for determining the structure of the trans-complex
J.-M.O., C.H.J., Y.H., S.K., J.-S.K., J.-Y.R., and J.S. performed research; J.Y.S., C.H.J., K.-J.Y.,J.-Y.R., and J.S.H. contributed new reagents/analytic tools; Y.Y., Y.-K.S., and D.-H.K.
and for providing answers to the questions of how fast fusion is
analyzed data; and T.-Y.Y., Y.-K.S., and D.-H.K. wrote the paper.
controlled in neurons and how the trans-complexes set up the
The authors declare no conflict of interest.
readily releasable vesicles with other regulatory proteins.
This article is a PNAS Direct Submission. J.R. is a guest editor invited by the Editorial Board.
Here, we show that certain small hydrophobic molecules
1Y.Y. and J.Y.S. contributed equally to this work.
(SHM) enable layer-by-layer control of SNARE zippering by
2To whom correspondence may be addressed. E-mail: or
wedging into various points of the SNARE zipper. SNARE-
mediated membrane fusion is dissected via this wedge-like action
This article contains supporting information online at
of SHMs. Analysis of the captured replication fork-like structure
PNAS December 21, 2010 vol. 107 no. 51 22145–22150



ControlKaempferol
Pre incubation control
Pre incubation control
K after preincubation
M after preincubation
K before preincubation
M before preincubation
Pre incubation control
Pre incubation control
D after preincubation
C after preincubation
D before preincubation
C before preincubation
SNARE complex formation (%)
Down-regulation of SNARE-driven membrane fusion by SHMs. (A) Percentage maximum fluorescence intensity was plotted as a function of time in
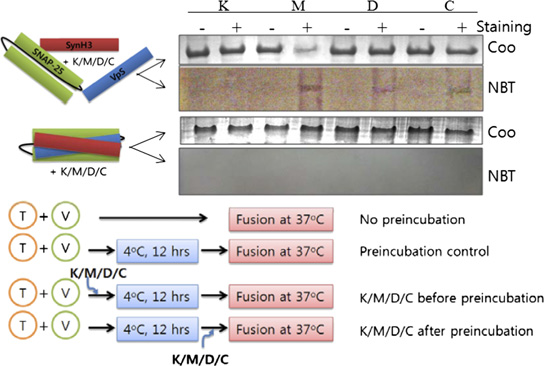
the presence or absence of SHMs. VpS, the soluble domain of VAMP2 lacking the transmembrane domain. SHMs were added at 10 μM concentration. (B)SNARE complex formation was assessed after the membrane-fusion assay by Western blotting using anti-SNAP25 antibody. (C) Correlation between thedegree of membrane fusion and the amount of SNARE complex formed in the presence or absence of SHMs. The concentration of SHM was 10 μM, and thedegree of fusion and complex formation were determined after an 80-min fusion initiation. Chemical structures of delphinidin, cyanidin, myricetin, andkaempferol are shown in the Inset. Concentration-dependent inhibition of fusion by these four SHMs is shown in (D) Soluble SNARE motifs form SDS-resistant complexes with SHMs in the inner layer. In the upper two gels, soluble fragments of SNARE proteins (each 500 μg/mL) were mixed with 10 μM SHMsand separated by SDS/PAGE. Gels were either stained with Coomassie blue (Coo) or electroblotted onto nitrocellulose membrane followed by incubation withnitroblue tetrazolium. + and − indicate the presence or absence of SHMs, respectively. In the lower two gels, the same experiment was performed withpreformed core complexes. Results indicate that SHMs bind to inner layer of the SNARE complex. K, kaempferol; M, myricetin; D, delphinidin; C, cyanidin. (E)Experimental procedures to determine fusion step-specific effects of SHMs on membrane fusion. (F) Fusion step-specific effects of SHMs on membrane fusion.
(G) Western blot analysis of SNARE complex formation. B, addition of SHMs before preincubation; A, addition of SHMs after preincubation.
We performed further experiments using the three most effi-
a slight effect (Fig. 1F). In contrast, when the SHMs were in-
cient compounds, delphinidin, cyanidin, and myricetin, as well as
troduced after the preincubation step, the effects became di-
the least effective, kaempferol (insets in Fig. 1C). First, by using
vergent. Myricetin largely retained its inhibitory activity, whereas
soluble SNARE motifs, SHMs were shown to intercalate into the
delphinidin and cyanidin showed little inhibitory effect. The
inner layer of the SNARE complex. SNARE complex-bound
amount of SNARE complex formed correlated well with the
SHMs were detected by nitroblue tetrazolium staining, which
results of the lipid-mixing assay (Fig. 1G).
allows colorimetric detection of protein-bound polyphenols, onlywhen the SHMs were added before complex formation, indicating
Binding Sites of SHMs in the SNARE Complex and the Effect of
that the inhibitory SHMs intercalate into the inner layer of the
Differential Binding on Hemifusion. We performed Ala-scanning
core complex during helical bundle formation (Fig. 1D). The
experiments to locate potential binding region of the three
SHMs did not bind to binary t-SNARE complex regardless of
SHMs in the SNARE zipper. All amino acids located at internal
the presence or absence of the Habc domain and transmembrane
a and d positions of the VAMP2 helix were mutated to alanine
domain of syntaxin 1
one at a time, with the exception of native alanine residues.
To gain further insight into the mechanism by which SHMs
P20A and P23A mutants were also prepared because the
inhibit membrane fusion, the fusion step-specific effect on
proline-rich region is known to have affinity to some polyphenols
SNARE-dependent proteoliposome fusion was examined (17).
(18), and VAMP2 contains the proline-rich region before the
Preincubation of t- and v-SNARE vesicle mixtures at 4 °C is known
SNARE motif. The effect of each Ala mutation was assessed
to enrich N-terminal, partially zipped complex and enhance
with the in vitro fusion assay (Fig. 2A). With only a few excep-
membrane fusion when the temperature is subsequently elevated
tions, most Ala mutations hampered SNARE-mediated fusion
to 37 °C. SHMs were added to the reaction either before or after
by ∼50%, emphasizing the importance of the conserved core
this preincubation step (Fig. 1E). SNARE-mediated fusion was
residues for the fusion activity. The Ala mutants were then
dramatically reduced when myricetin, delphinidin, or cyanidin was
subjected to the lipid-mixing assay in the presence of an SHM.
added before the preincubation step, whereas kaempferol had only
We reasoned that when a binding site for a particular SHM is

P2 P2 L3 T3 V39 A V42 A M4 N49 A V53 A R56 A L6 L6 L7 F7 L8 Y88 A K9
P2 P2 L3 T3 V39 A V42 A M4 N49 A V53 A R56 A L6 L6 L7 F7 L8 Y88 A K9
P2 P2 L3 T3 V39 A V42 A M4 N49 A V53 A R56 A L6 L6 L7 F7 L8 Y88 A K9
P2 P2 L3 T3 V39 A V42 A M46 A N49 A V53 A R56 A L6 L6 L7 F7 L8 Y88 A K9
000 2000 3000 4000
000 2000 3000 4000
Determination of SHM wedging points in the SNARE zipper and correlation of the degree of zippering with membrane-fusion stage. (A) The effect of
alanine mutations on relative fusion efficiency. RFmo denotes the relative fusion efficiency of alanine mutants compared with wild-type VAMP2. RFmi is theratio between percentage maximum fluorescence intensities obtained in the presence or absence of inhibitors for the same mutant, where the inhibitors aremyricetin (M), delphinidin (D), and cyanidin (C) from left to right. The mutations with significantly higher values (blue bars) above RFwi (black bars) indicatethat those mutations are not inhibited by the SHM and thus represent the points that the SHM wedges into the SNARE zipper. The first black bar in each setdenotes RFwi for each SHM. Equations used to obtain relative fusion efficiency values are described in (B) Percentage hemifusion state was determinedthrough the inner-leaflet mixing assay in the absence or presence of SHMs. The actual time-dependent traces are shown in each SHM was used at10 μM in both studies.
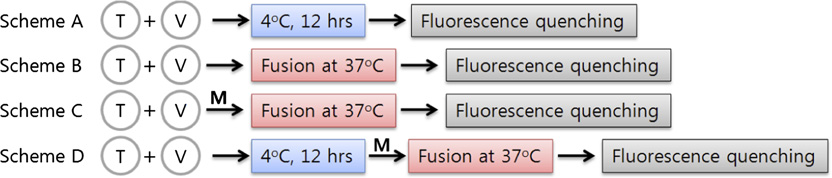
mutated to Ala, the mutant would lose ability to bind the SHM,
fluorescence-quenching assays were performed with Cy5-labeled
thereby relieving inhibition by that SHM The relative
VAMP2 mutants (Fig. 3). Five Cy5-labeled VAMP2 mutants
fusion efficiency, defined as the ratio between the percentage
were prepared: Q33C and T35C to probe N-terminal zippering,
maximum fluorescence intensity (PMF) measured in the pres-
L54C and R56C for middle region zippering, and D68C for
ence of the drug to the PMF measured in the absence of the
C-terminal zippering. FO/F, where FO and F represent fluores-
drug, was plotted along the VAMP2 helix sequence for each
cence intensity measured in the absence or presence of the fluo-
SHM. Myricetin appears to have two binding sites: a weak
rescence quencher acrylamide, respectively, increases if acrylamide
binding site coordinated by hydrophobic residues between resi-
is readily accessible to Cy5, indicating the labeled position is frayed.
dues 20 and 35 of VAMP2 and the corresponding t-SNARE
Conversely, the slope is less affected by the quencher when the
hydrophobic layers and a strong binding site coordinated by the
Cy5-label is positioned in the coiled-coil region. In this regard,
hydrophobic layers in the middle region (residues 49–63 of
preincubation at 4 °C (Fig. 3, scheme A) seems to result in the
VAMP2 and the corresponding t-SNARE residues). In contrast,
replication fork-like SNARE zipper, where the N-terminal region
delphinidin and cyanidin both had only one strong binding site at
of VAMP2 is in the coiled-coil structure and the C-terminal region
the N-terminal region between residues 20 and 42. These results
remains frayed. After membrane fusion at 37 °C (Fig. 3, scheme B),
were consistent with the results from the preincubation experi-
all labeled VAMP2 residues appeared to participate in SNARE
ments. It is noted that the proline-rich region also contributes to
complex formation. By contrast, in the presence of myricetin
SHM binding to the N terminus of SNARE complex, perhaps
(Fig. 3, scheme D), the fluorescence-quenching pattern was similar
through hydrophobic interaction (18). Although high-resolution
to those of scheme A, indicating that SNARE zipper wedged by
structures of SHM-containing SNARE complexes need to be
myricetin has the frayed C terminus.
determined for better understanding of binding mechanisms,we speculate that the proline-rich region caps the N-terminal
Dissection of SNARE-Dependent Membrane Fusion in PC12 Cells with
SNARE bundle to enhance the SHM binding and
SHMs. The next natural question is whether these observations are
also true in live cells. First, we tested whether the SHMs could
We hypothesized that it would be possible to link the degree of
regulate SNARE complex formation in live cells. We prepared
SNARE zippering to specific stages of membrane fusion by har-
NGF-treated PC12 cells loaded with [3H]noradrenaline. Based
nessing the unique properties of each SHM. We measured hem-
on the in vitro results, we expected that the SHM binding sites in
ifusion by using sodium dithionite that selectively kills fluorescent
the SNARE complexes would only be exposed during recycling of
dyes in outer leaflets (19). Myricetin inhibited inner-leaflet mixing
SNARE proteins. To induce SNARE recycling, the cells were
efficiently but allowed outer-leaflet mixing, stalling as much as
pretreated with a high concentration of KCl followed by imme-
80% of the fusion outcome at the hemifusion state (Fig. 2B). In
diate addition of SHMs. A high concentration of KCl is known to
contrast, delphinidin and cyanidin had just a slight effect on the
induce depolarization of PC12 cells leading to SNARE-mediated
fraction of hemifusion (Fig. 2B and Together, our results
membrane fusion and release of neurotransmitters (20).
raise the possibility that myricetin arrests membrane fusion at
After high-K+ pretreatment (Fig. 4A, red box), neurotrans-
the hemifusion state by wedging into the middle region of the
mitter release was strongly inhibited by SHM exposure (Fig. 4B,
SNARE zipper.
red bars). The three most competent compounds, delphinidin,
cyanidin, and myricetin, showed 75–80% inhibition at 10 μM,
Overall Fold of the Half-Zipped SNARE Complex. To determine
comparable to the calcium channel blocker verapamil (21, 22) and
the rough structure of SNARE zipper wedged by myricetin,
only slightly lower than the Clostridium botulinum neurotoxin D
PNAS December 21, 2010 vol. 107 no. 51 22147

V only (Q33C)
V only (T35C)
V (Q33C) + T
V (T35C) + T
V only (L54C)
V only (R56C)
V (L54C) + T
V (
V only (D68C)
V (D68C) + T
[Acrylamide, mM]
[Acrylamide, mM]
[Acrylamide, mM]
[Acrylamide, mM]
Fluorescence-quenching experiments for Cy5-labeled VAMP2 mutants. (A) Schematic presentation of fluorescence-quenching experiments to de-
termine the structure of the SNARE zipper wedged by myricetin. (B) Stern–Volmer plots for the five Cy5-labeled VAMP2 mutants measured under variousconditions as indicated in schemes A–D. FO and F represent fluorescence intensity measured in the absence or presence of the fluorescence quencheracrylamide, respectively. Arrows indicate that the residues are engaged into the coiled-coil region when t-vesicle is present. A smaller difference in quenchingefficiency (indicated by short arrows) may result from partial coiling (schemes A and D) or because of a mixture of free VAMP2 and the half-zippered complex(scheme C). (C) Structures of SNARE zipper expected from the fluorescence-quenching experiments.
(BoNT/D). This high-K+ pretreatment experiment appeared
binding sites are concealed inside the N-terminal bundle and the
to mirror the in vitro preincubation experiment in which SHMs
C-terminal stronger myricetin-binding site is not yet zipped.
were added before 4 °C preincubation (Fig. 1 E and F). Alter-
natively, the in vitro results of adding SHMs after 4 °C pre-incubation were expected to correspond to those from the
In the present study, it was shown that dynamic SNARE zip-
neuronal cells without high-K+ pretreatment (Fig. 4A, blue box).
pering process could be stopped at the point of interest by
Only myricetin showed a significant inhibitory effect on neuro-
wedging an appropriate SHM into the zipper. Delphinidin andcyanidin wedge into the SNARE zipper at the far N terminus
exocytosis (Fig. 4B, blue bars), which is consistent with our in vitro
(∼P20–V42 of VAMP2), thereby preventing N-terminal nucle-
observations that myricetin hampered C-terminal zippering by
ation of SNARE complex formation (Myricetin stops
wedging the half-zipped partial complex.
subsequent C-terminal zippering by wedging into the middle
Membrane proteins were extracted from PC12 cells and ana-
region of the SNARE zipper after the N-terminal half is zipped
lyzed by Western blotting using an antibody against SNAP-25 (Fig.
By harnessing these unique characteristics of SHMs,
4C). Depolarization by high K+ indeed induced SDS-resistant
we dissected SNARE-driven membrane fusion and tried to link
SNARE complex formation (low K+ vs. high K+). In the presence
the degree of SNARE zippering to the specific membrane-
of SHMs, depolarization-induced SNARE complex formation was
reduced, which is in agreement with the neurotransmitter release
Preincubation at 4 °C is known to enhance membrane fusion
results. Delphinidin and cyanidin effectively reduced SNARE
at the subsequently elevated temperature. This preincubation
complex formation only when the neuronal cells were pretreated
step is thought to accumulate the N-terminal partial complexes
with high-K+ solution (Fig. 4C, red box). In contrast, myricetin
), which undergo complete zippering in a synchronized
effectively reduced SDS-resistant complex formation even without
manner when the temperature is raised (11). When t-vesicles
high-K+ pretreatment (Fig. 4C, blue box). Thus, the conformation
were mixed with vesicles containing Cy5-labeled VAMP2 at 4 °C,
of the trans-SNARE complex in neuronal cells is likely to be similar
we could confirm the formation of the N-terminal partial com-
to that observed in vitro, where the delphinidin- and cyanidin-
plex with frayed C-terminal SNARE motifs (Fig. 3). The partial
The effect of SHMs on neurotransmitter release and SNARE complex formation. (A) Experimental procedures. Red box, high-K+ pretreatment; blue
box, no pretreatment. (B and C) After 15 min of main high-K+-induced depolarization, the amount of released neurotransmitters (B) and the amount of SDS-resistant SNARE complexes (C) were quantified in the supernatant and membrane fraction, respectively. α, β, and γ indicate the time of sampling formembrane fraction as shown in A. Reduced band intensity indicated by γ compared with β in the red-boxed gel shows that the pretreatment of high K+effectively dissembles preexisting SDS-resistant SNARE complexes in the cells. Red, high-K+ pretreatment group; blue, no pretreatment group. The effect ofSHMs on cell viability is shown in . IC50 values of SHMs on neurotransmitter release are shown in . Statistical significance of differenceswas evaluated by ANOVA (*P < 0.05).
complex, however, has never been captured at physiological
Evidence indicates that the hemifusion state is a stable in-
temperatures. The arrested zippering at physiological tempera-
termediate of exocytosis in neuronal cells in vivo (28), and two
ture by myricetin provides information about the trans-complex.
membranes may be hemifused before Ca2+ influx (29, 30). The
Because the myricetin binding site is predicted to be coordinated
similar responses to SHM treatments observed in both recon-
by the hydrophobic residues of VAMP2 ∼N49–L63 and corre-
stituted proteoliposomes and neuronal PC12 cells suggest that
sponding t-SNARE residues (Fig. 2A), and the partial complex
the halfway zippering-mediated hemifusion state (may
formed by 4 °C preincubation is susceptible to myricetin (Fig. 1
be also true in the neuron. The hemifusion state likely progresses
E–G), the results suggested that the region of VAMP2 after N49
toward the fusion pore when calcium triggers full SNARE zip-
remains unzipped in the partial complex. Because this partial
pering ), with the help of other regulatory proteins such
complex is not susceptible to delphinidin or cyanidin, which bind
as synaptotagmin and complexin (31). We note, however, thatthere are contradictory results against the hemifusion state (32),
before V42, the SHMs-assisted assays suggest the possibility that
and there is the possibility, although unlikely, that myricetin's
the N-terminal region up to M46 forms a coiled coil during 4 °C
interaction with the membrane is in part responsible for hemi-
preincubation. Static fluorescence-quenching experiments (Fig.
3) also support that this halfway N-terminal zippering up to M46
BoNTs elicit neuron-specific flaccid paralysis by specifically
with the frayed C-terminal half of VAMP2 is likely to represent
cleaving neuronal SNARE proteins. After the realization of the
the trans-complex ).
therapeutic potentials of these otherwise fatal toxins, the Food
It is believed that SNARE complex formation starts at the
and Drug Administration approved the controlled use of BoNT/
membrane-distal N-terminal region and zips toward the mem-
A for the treatment of several hypersecretion-related neurolog-
brane-proximal C-terminal region (6–9), thereby effectively forc-
ical diseases, such as strabismus, blepharospam, hemifacial
ing the apposition of the two membranes. Also, it is widely ac-
spasm, and cervical dystonia (33). Recently, the cosmetic use of
cepted that N-terminal assembly of SNARE proteins underlies
these toxins for treating glabellar facial lines and axillary hy-
vesicle priming, whereas assembly of the C-terminal end drives
perhidrosis has gained immense popularity. Can small molecules,
fusion. In this model, an intermediate state of the SNARE com-
with more conventional drug-like properties, also regulate
plex is associated with the primed vesicle state, and subsequent
SNARE complex formation and consequent neuroexocytosis?
C-terminal assembly, assisted by other proteins such as synapto-
Promisingly, our results imply that SNARE-wedging small mol-
tagmin and complexin, leads to membrane fusion and the re-
ecules might serve as SNARE-specific drugs, which will definitely
laxation of trans-SNAREpins into cis-SNARE complexes. Many
provide a tractable avenue in treating a variety of human hy-
of recent studies on complexin- and synaptotagmin-modulated
persecretion diseases. These small molecules can serve as gen-
fast neuroexocytosis presume this model to be true to account for
eral chemical platforms from which unique and potent SNARE-
their findings (3, 10, 23–27). However, our results raise another
specific drugs can be derived.
possibility, where N-terminal half zippering, which had been
Materials and Methods
merely considered responsible for vesicle docking, might be suf-
cient to induce hemifusion and is the minimal zippering degree
for membrane hemifusion. Our results suggest that the zippering
degree responsible for hemifusion could be only up to residue 46
sn-glycero-3-phosphoethanolamine-N-(lissamine rhodamine B sulfonyl)
(rhodamine-PE) were obtained from Avanti Polar Lipids. RPMI medium 1640,
PNAS December 21, 2010 vol. 107 no. 51 22149
penicillin-streptomycin, horse serum, and FBS were purchased from GIBCO/
ferred to scintillation vials and measured by liquid scintillation counting. The
BRL. Recombinant BoNT/D light chain was purchased from List Biological
quantity of released neurotransmitter was calculated according to the fol-
Laboratories. All other chemicals, including polyphenolic compounds, were
lowing equation: The quantity of released [3H]noradrenaline = (cpm of high-
purchased from Sigma-Aldrich.
K+-stimulated sample − cpm of basal level release)/mg of protein. The amountof secreted neurotransmitters was determined without pretreatment of
PC12 Cell Culture and Determination of [3H]Noradrenaline Release. PC12
detergents for permeabilization except for VpS and BoNT/D. Cell per-
cells were purchased from the Korean Cell Line Bank (Seoul, Korea). The PC12
meabilization for incorporation of VpS and BoNT/D (5 nM) was accomplished
cells were plated onto poly-D-lysine–coated culture dishes and were main-
with 10 μM digitonin in low-K+ solution (34). For high-K+ pretreatment groups,
tained in RPMI medium 1640 containing 100 μg/mL streptomycin, 100 U/mL
polyphenols were not added before the first high-K+ treatment but were
penicillin, 2 mM L-glutamine, and 10% heat-inactivated FBS at 37 °C ina 5% CO
before the second high-K+ treatment.
2 incubator. The cell cultures were split once a week, and the me-
dium was refreshed three times a week. PC12 cells were treated withNGF (7S, 50 ng/mL, Invitrogen) for 5 d before uptake and release of [3H]
Statistical Analysis. All experimental data were examined by ANOVA pro-
cedures, and significant differences among the means from three to five
After NGF-treated PC12 cells were grown in 12-well plates at a density of
repeats were assessed with Duncan's multiple range tests and the Statistical
4 × 105 cells per dish, small-molecule compounds (10 μM) and [3H]noradren-
Analysis System, version 8.2 (SAS Institute).
aline (1 μCi/mL) were applied for 10 min in low-K+ solution (140 mM NaCl, 4.7mM KCl, 1.2 mM KH2PO4, 2.5 mM CaCl2, 1.2 mM MgSO4, 11 mM glucose, and
ACKNOWLEDGMENTS. This work was supported by the Basic Science Re-
15 mM Hepes-Tris, pH 7.4). The cells were washed four times to remove the
search Program (2007-D00243 and 2010-0015035), by the Mid-Career Re-
unincorporated radiolabeled neurotransmitters. The cells were then depo-
searcher Program (2009-0058612), and by the World Class University Program
larized with a high-K+ solution (115 mM NaCl, 50 mM KCl, 1.2 mM KH2PO4,
through the National Research Foundation of Korea. This work was also
2.5 mM CaCl2, 1.2 mM MgSO4, 11 mM glucose, and 15 mM Hepes-Tris, pH 7.4)
supported by Amorepacific Corporation and Small and Medium Business
for 15 min to assess the stimulated release. Extracellular media was trans-
1. Söllner T, et al. (1993) SNAP receptors implicated in vesicle targeting and fusion.
19. Lu X, Zhang F, McNew JA, Shin YK (2005) Membrane fusion induced by neuronal
SNAREs transits through hemifusion. J Biol Chem 280:30538–30541.
2. Rizo J, Rosenmund C (2008) Synaptic vesicle fusion. Nat Struct Mol Biol 15:665–674.
20. Ray P, Berman JD, Middleton W, Brendle J (1993) Botulinum toxin inhibits arachidonic
3. Südhof TC, Rothman JE (2009) Membrane fusion: Grappling with SNARE and SM
acid release associated with acetylcholine release from PC12 cells. J Biol Chem 268:
proteins. Science 323:474–477.
4. McNew JA (2008) Regulation of SNARE-mediated membrane fusion during exocytosis.
21. Hay DW, Wadsworth RM (1983) The effects of calcium channel inhibitors and other
Chem Rev 108:1669–1686.
procedures affecting calcium translocation on drug-induced rhythmic contractions in
5. Sørensen JB (2005) SNARE complexes prepare for membrane fusion. Trends Neurosci
the rat vas deferens. Br J Pharmacol 79:347–362.
22. Richardson CM, Dowdall MJ, Bowman D (1996) Inhibition of acetylcholine release
6. Fiebig KM, Rice LM, Pollock E, Brunger AT (1999) Folding intermediates of SNARE
from presynaptic terminals of skate electric organ by calcium channel antagonists: A
complex assembly. Nat Struct Biol 6:117–123.
detailed pharmacological study. Neuropharmacology 35:1537–1546.
7. Chen YA, Scales SJ, Scheller RH (2001) Sequential SNARE assembly underlies priming
23. McMahon HT, Kozlov MM, Martens S (2010) Membrane curvature in synaptic vesicle
and triggering of exocytosis. Neuron 30:161–170.
fusion and beyond. Cell 140:601–605.
8. Melia TJ, et al. (2002) Regulation of membrane fusion by the membrane-proximal coil
24. Rizo J (2010) Synaptotagmin-SNARE coupling enlightened. Nat Struct Mol Biol 17:
of the t-SNARE during zippering of SNAREpins. J Cell Biol 158:929–940.
9. Ellena JF, et al. (2009) Dynamic structure of lipid-bound synaptobrevin suggests
25. Matos MF, Mukherjee K, Chen X, Rizo J, Südhof TC (2003) Evidence for SNARE
a nucleation-propagation mechanism for trans-SNARE complex formation. Proc Natl
zippering during Ca2+-triggered exocytosis in PC12 cells. Neuropharmacology 45:
Acad Sci USA 106:20306–20311.
10. Stein A, Weber G, Wahl MC, Jahn R (2009) Helical extension of the neuronal SNARE
26. Maximov A, Tang J, Yang X, Pang ZP, Südhof TC (2009) Complexin controls the force
complex into the membrane. Nature 460:525–528.
transfer from SNARE complexes to membranes in fusion. Science 323:516–521.
11. Weber T, et al. (1998) SNAREpins: Minimal machinery for membrane fusion. Cell 92:
27. Xue M, et al. (2010) Binding of the complexin N terminus to the SNARE complex
potentiates synaptic-vesicle fusogenicity. Nat Struct Mol Biol 17:568–575.
12. Liu S, Wu S, Jiang S (2007) HIV entry inhibitors targeting gp41: From polypeptides to
28. Wong JL, Koppel DE, Cowan AE, Wessel GM (2007) Membrane hemifusion is a stable
small-molecule compounds. Curr Pharm Des 13:143–162.
intermediate of exocytosis. Dev Cell 12:653–659.
13. Cai L, Gochin M (2007) A novel fluorescence intensity screening assay identifies new
29. Zampighi GA, et al. (2006) Conical electron tomography of a chemical synapse:
low-molecular-weight inhibitors of the gp41 coiled-coil domain of human immuno-
Vesicles docked to the active zone are hemi-fused. Biophys J 91:2910–2918.
deficiency virus type 1. Antimicrob Agents Chemother 51:2388–2395.
30. Vrljic M, et al. (2010) Molecular mechanism of the synaptotagmin-SNARE interaction
14. Frey G, et al. (2006) Small molecules that bind the inner core of gp41 and inhibit HIV
in Ca2+-triggered vesicle fusion. Nat Struct Mol Biol 17:325–331.
envelope-mediated fusion. Proc Natl Acad Sci USA 103:13938–13943.
31. Schaub JR, Lu X, Doneske B, Shin YK, McNew JA (2006) Hemifusion arrest by
15. Cooper WJ, Waters ML (2005) Molecular recognition with designed peptides and
complexin is relieved by Ca2+-synaptotagmin I. Nat Struct Mol Biol 13:748–750.
proteins. Curr Opin Chem Biol 9:627–631.
32. Fernández-Busnadiego R, et al. (2010) Quantitative analysis of the native presynaptic
16. Otto H, Hanson PI, Jahn R (1997) Assembly and disassembly of a ternary complex of
cytomatrix by cryoelectron tomography. J Cell Biol 188:145–156.
synaptobrevin, syntaxin, and SNAP-25 in the membrane of synaptic vesicles. Proc Natl
33. Dolly JO, Lawrence GW, Meng J, Wang J, Ovsepian SV (2009) Neuro-exocytosis:
Acad Sci USA 94:6197–6201.
Botulinum toxins as inhibitory probes and versatile therapeutics. Curr Opin Pharmacol
17. Shen J, Tareste DC, Paumet F, Rothman JE, Melia TJ (2007) Selective activation of
cognate SNAREpins by Sec1/Munc18 proteins. Cell 128:183–195.
34. Jung CH, et al. (2008) A search for synthetic peptides that inhibit soluble N-
18. Hagerman AE, Butler LG (1981) The specificity of proanthocyanidin-protein inter-
ethylmaleimide sensitive-factor attachment receptor-mediated membrane fusion.
actions. J Biol Chem 256:4494–4497.
FEBS J 275:3051–3063.
Source: http://yoonlab.yonsei.ac.kr/Publications/22145.full.pdf
diabetes.co.uk
Scientific Research Scientific Information for Health Care Professionals Almased UK Ltd.2nd Floor Berkeley Square HouseBerkeley SquareLondon W1J 6BD Phone: 020 7969 1886Email: [email protected]: www.almased.co.uk The most important studies on the effects of Almased® Table of Contents 4 Editorial 20 "Supported insulin and blood sugar
smar.ma
Pédiatrie 1 P1- Antibiothérapie probabiliste en milieu de réanimation pédiatrique O.EL ALLAM, Y.HARTI, Y.ALAOUI, B.HMAMOUCHI, S.NEJMI, A.CHLILEK SERVICE DE REANIMATION PEDIATRIQUE POLYVALENTE CHU IBN ROCHD DE CASABLANCA Introduction : L'antibiothérapie probabiliste correspond à une prescription d'antibiotiques réalisée avant de connaitre la nature et la sensibilité des germes responsable de l'infection. En pédiatrie l'évolution d'un processus infectieux sévère est souvent plus rapide que chez l'adulte, avec le risque d'apparition souvent précoce d'une insuffisance circulatoire. Le but de notre travail est la description et l'évaluation de l'antibiothérapie probabiliste en milieu de réanimation pédiatrique polyvalente CHU Ibn Rochd de Casablanca. Patients et méthodes : Etude rétrospective étalée sur 11 mois de janvier 2012 à novembre 2012 qui a permis le recrutement de 142 patients. Les données recueillies sont les critères épidémiologiques des patients, les antécédents médicaux, la notion de colonisation bactérienne, le type d'infection motivant l'introduction de l'antibiothérapie probabiliste, les circonstances du choix de l'antibiotique, le caractère précoce ou tardif et la durée de l'antibiothérapie probabiliste, le retentissement du changement de l'antibiothérapie sur le pronostic, l'évolution et la durée de séjour. Résultats : L'âge moyen était de 37,44 mois, le poids moyen était de 13,28kg, 7,7%des patients avaient des antécédents cardiaques, 4,2% avaient des antécédents respiratoires, 1,4% avaient un déficit immunitaire, 1,4% étaient anciens prématurés, 83,1% des patients étaient hospitalisés antérieurement avec notion de prise d'antibiotiques dans 11,3% des cas. 64,8% de nos patients avaient une infection pulmonaire, 9,2% avaient une infection urinaire, 13,4% avaient une infection neuromeningée, 15,5% une septicémie. L'antibiothérapie probabiliste prescrite était à base d'une monothérapie dans 11,5% des cas, une bithérapie dans 59,8% des cas et une trithérapie dans 28,7% des cas avec le choix du ceftriaxone dans 60,5% des cas. L'heure de début de l'antibiothérapie était le jour dans 52,8% des cas, la nuit dans 44,4% et le weekend dans 2,8% des cas. La décision était prise par un médecin junior dans 54,2% des cas et un médecin seigneur dans 45,8% des cas avec un changement de cette antibiothérapie selon la gravité dans 38% des cas et selon la bactériologie dans 21,8% des cas. La durée moyenne de l'antibiothérapie probabiliste était de 10,79 jours. L'évolution était favorable dans 66,2% des cas avec un taux de mortalité de 33,8%. Conclusion : La prescription raisonnée de l'antibiothérapie probabiliste initiale a démontré son impact sur l'amélioration du pronostic vital des patients. Le caractère nosocomial ou communautaire de l'infection, la connaissance de l'écologie bactérienne du service où l'on travaille, de la flore colonisante du patient et des données de l'examen direct des prélèvements bactériologiques jouent un rôle majeur dans cette décision.