Chapter 14 veterinary aspects
COMMISSIONED PAPER (UK) This paper was commissioned by FECAVA for the Special issue of EJCAP,
Genetic/Hereditary Disease and Breeding. Must not be copied without permission 2014
Chiari–like malformation and syringomyelia
Clare Rusbridge
Introduction
Syringomyelia is a condition characterised by fluid filed cavities (syrinxes or syringes) within the central spinal
cord and the resulting damage produces clinical signs of pain and neurological deficits. Since the increase in
availability of magnetic resonance imaging (MRI), syringomyelia is an increasingly common diagnosis in
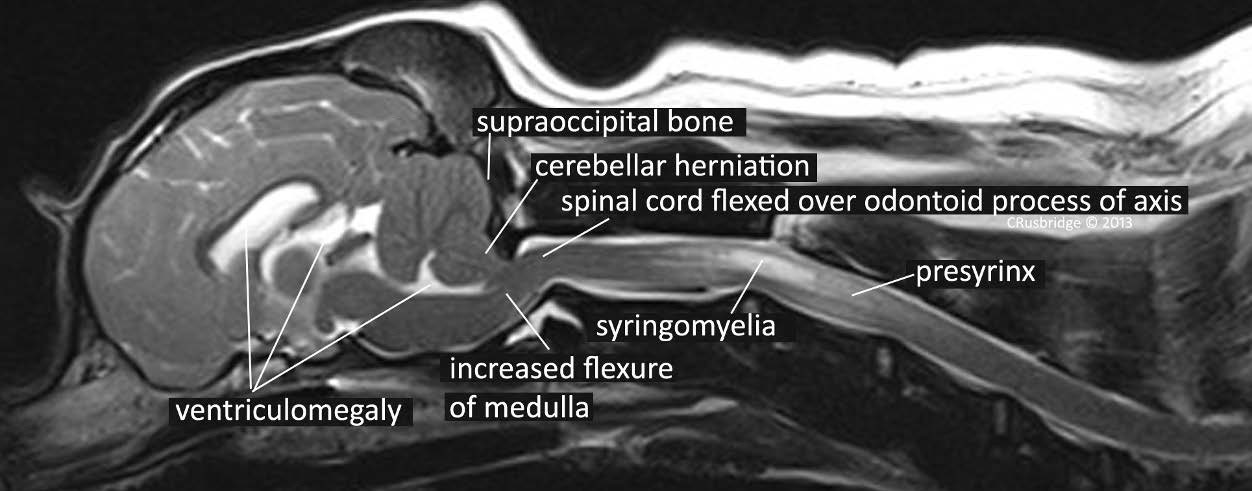
veterinary medicine (1, 2) The most common cause of syringomyelia in the dog is Chiari-like malformation (Fig
1), a condition analogous to Chiari Type I and 0 malformation in humans (3, 4).
Figure 1 Midline sagittal T2-weighted MRI images of the brain and cervical spinal cord from 1 year old female
CKCS with Chiari malformation and syringomyelia and presenting with pain.
Pathophysiology of syringomyelia
A satisfactory explanation of how syringomyelia develops has yet to be elucidated. There is not even a
consensus as to whether syrinx fluid is derived from extracellular or cerebrospinal fluid (CSF) (5-8)].
Syringomyelia is a disorder of CSF and therefore understanding the pathogenesis of this enigmatic disorder is
dependent on understanding CSF flow dynamics, biochemistry and factors that influence its absorption and
The majority of CSF is produced by the four choroid plexuses (one in each ventricle of the brain), which
circulates through the ventricular system and the subarachnoid spaces of the brain and spinal cord (9, 10).
Drainage of CSF is partly into the blood through arachnoid granulations and vil i and partly along lymphatic
drainage pathways, mostly associated with the cribriform plate of the ethmoid bone (11). It has also been
suggested that the spinal central canal may play a part in drainage of CSF and/or excess extracel ular fluid as
there is functional communication between the central canal and the subarachnoid space at the terminal
ventricle (12, 13) . One of the major functions of CSF is as a mechanical buffer however it does not just provide
a physical cushion and reduces tension on nerve roots but also accommodates the pressure of the systolic
pulse and reduces the weight of this heavy organ. Without the CSF a human could not stand upright and within
the CSF a 1500g brain weighs only 50g (14) .
According to the Munro-Kelie doctrine the central nervous system and its accompanying fluids are enclosed in
a rigid container whose total volume remains constant. Therefore when the heart beats and there is increase
in volume of intracranial blood, CSF is displaced from the cranial to the spinal subarachnoid space through the
foramen magnum thus avoiding a deleterious increase in intracranial pressure. The spinal dural sac is
distensible, further increasing the compliance of the system and minimising rises in central nervous system
pressure (15). Disturbance of the normal free flow of CSF through the foramen magnum appears to be a major
factor responsible for the formation of a syrinx in the cervical spinal cord (2, 16, 17). However there may be
other possible factors influencing the pathogenesis of a syringomyelia such as failure of absorption or drainage
of extracel ular fluid (18), intracranial hypertension (19-21), imbalance in the production and absorption of CSF
(22) disruptions of the blood-spinal cord barrier or alterations of aquaporin expression (23) . The currently
most accepted theory of pathogenesis of syringomyelia is that obstruction to CSF flow in the subarachnoid
space results in a mismatch in timing between the arterial pulse peak pressure and CSF pulse peak pressure.
Earlier arrival of peak CSF pressure compared to peak spinal arterial pressure encourages flow of CSF into the
perivascular space. The perivascular space changes in size during the cardiac cycle and is widest when spinal
arteriole pressure is low. If at that time peak CSF pressure is high then the perivascular space could act as a
‘leaky' one-way valve (8, 24-27). From the perivascular space, fluid flows into the central canal ultimately
resulting in a syrinx (28-30). However this theory also leaves many unanswered questions and further study is
In the dog syringomyelia is associated with a number of different pathologies with a common theme of CSF flow
obstruction. The most common cause is Chiari-like malformation, which is a complex abnormality characterised
by overcrowding of the craniocervical junction and obstruction of CSF flow through the foramen magnum. It is
unclear why some dogs with Chiari-like malformation develop syringomyelia and some do not (31, 32) .
Numerous studies, mostly in Cavalier King Charles spaniel (CKCS) and Griffon Bruxel ois (Table 1) have identified
many "pieces of the jigsaw" however key parts are stil missing. No study has identified a single anatomical
feature that consistently predicts syrinx development and it is likely that the pathogenesis of syringomyelia is a
multifactorial process.
Table 1. Pathogenesis of Chiari-like malformation and syringomyelia: summary of the existing knowledge base.
Abbreviations used in tables: CKCS – Cavalier King Charles spaniel; SM – syringomyelia; CM – Chiari-like
malformation. CCD – central canal dilatation.
Anatomical feature Study Finding(s)
Significance relating to
Reference
Brachiocephalic breeds have
early closure of the spheno-
Premature closure of the spheno-
occipital synchondrosis. In CKCS
occipital synchondrosis wil result
closure is even earlier
in a short cranial base predisposing
CKCS have shorter braincase in
brain overcrowding
Brachycephalicism
relation to width compared to
other brachycephalic dog breeds
Griffon Bruxel ois with CM have
Basiocranial shortening results in
shortened basicranium and
supraoccipital bone, with a
changes in the rostral cranial fossa
compensatory lengthening of the but caudal cranial fossa
cranial vault, especial y the
overcrowding persists
CKCS with CM and SM have a
Smal er caudal cranial fossa volume
shal ower and smal er volume
predisposes caudal cranial fossa
caudal cranial fossa compared to
Caudal cranial fossa
CKCS with CM only and other
CKCS have a strong relationship
Smal breed dogs and Labrador
between hindbrain volume and
retrievers compensate for
volume of the rostral part of the
variations in hindbrain volume by
caudal cranial fossa and a weak
modifying growth of the occipital
relationship between hindbrain
skul . In the CKCS, increased
volume and volume of the caudal cerebel ar size is not
part of the caudal cranial fossa. In accommodated by increased
Labrador retrievers and other
occipital bone development and
smal breed dogs this relationship the tentorium cerebel i
compensates by bulging in a rostral
The absolute and relative volume Mismatch in skul and brain volume (39)]
of the CKCS skul is similar to
is associated with development of
other brachycephalic toy dog
breeds but CKCS have a greater
volume of parenchyma within the
Parenchymal (brain)
caudal cranial fossa.
CKCS with early onset SM have a
larger volume of parenchyma
within a smal er caudal cranial
fossa compared to older CKCS
CKCS have relatively increased
cerebel ar volume compared to
other control breeds and this is
associated with development of
Caudal cranial fossa overcrowding
Cerebel ar volume
is associated with development of
Increased cerebel ar volume in
CKCS is correlated with increased
crowding of the cerebel um in the
caudal part of the caudal cranial
Obstruction of CSF channels
Commonly seen but presence or
though the foramen magnum
size does not predict SM
contributes to the pathogenesis of
SM but there must also be other
predisposing factors.
Positive association with the size
Cerebel ar herniation
of foramen magnum and size of
Overcrowding of the caudal cranial (31)]
cerebel ar herniation
fossa causes supraoccipital bone
resorption (occipital dysplasia)
The length of the cerebel ar
herniation increases with time
The size of the foramen magnum
Cerebel ar pulsation
CKCS with CM and SM have
Abnormal cerebel ar pulsation
significantly greater pulsation of
could lead to a mismatch in the
the cerebel um compared to
timing of the arterial and CSF pulse
CKCS with CM only and other
waves predisposing SM
Higher peak CSF flow velocity at
the foramen magnum with a
lower CSF flow velocity at C2–C3
Alterations in the CSF velocity
profile predispose SM
Turbulence at the foramen
magnum and at the C2–C3 disc
significantly associated with SM
In CKCS ventricle dimensions are
SM is related to CSF disturbances
positively correlated with syrinx
Ventricle dimensions
Are not correlated with seizures
Epilepsy and CM in CKCS should be
(nor is caudal cranial fossa
considered unrelated
CKCS with CM and SM have
Venous narrowing at the jugular
narrowed jugular foramina in
foramina associated with smal
comparison with CKCS with CM
skul base can lead to elevated
Jugular foramina
venous pressure and impaired CSF
CKCS with CM and SM have
Reduced venous sinus volume
reduced venous sinus volume in
could result in intracranial
Venous sinus volume
comparison with CKCS with CM
hypertension and impaired CSF
In CKCS, SM tends to develop first According to the Venturi effect,
within the C2–C4, T2-T4 and T12- increased fluid velocity through a
L2 spinal-cord segments. These
narrowed flow channel decreases
are regions where the
hydrostatic pressure in the fluid,
subarachnoid space narrows
meaning that there may be a
and/or there is a change in the
tendency for the spinal cord to be
angulation of the vertebral canal
"sucked" outward in these regions
which may contribute towards SM.
However other studies have
suggested that the contribution of
the Venturi effect is insignificant
In CKCS 76% of dogs with a syrinx In CKCS MRI imaging of the cranial
at C1-C4 also had a syrinx in the
cervical region only has high
C5-T1 and T2-L2 regions and 49%
sensitivity for detection of SM
had a syrinx in the L3-L7 region
however the extent of the disease
may be underestimated
Occasional comorbidity with CM
No significant association with SM
Size of C2 spinous
Significantly smaller in CKCSs
than in non-CKCS breeds
Commonly seen in association
occipital overlapping
with CM especial y in non-CKCS
Additional compression of CSF
channels may contribute to
Dorsal impingement
Commonly seen in association
development of SM but a
subarachnoid space /
consistent association has not been (31, 36, 52)]
spinal cord at C1-C2
Ventral impingement
Commonly seen in association
of subarachnoid space with CM (Fig 1)
(31, 36, 52, 54)]
/ neural tissue by dens
Increased width of spinal canal at
Width of spinal canal
C2- C3 and C3 in CKCS with SM
Questionable clinical significance
Angulation at C2-C3
Pain is positively correlated with
Dogs with a wider asymmetrical
SM transverse width and
SM more likely to experience
symmetry on the vertical axis,
A syndrome of neuropathic pain is
more likely when there is
asymmetrical dorsal horn
Prevalence and incidence
Chiari malformation
Brachycephalicism and miniaturisation are risk factors for Chiari-like malformation (35). The condition is most
commonly reported in toy breed dogs, in particular CKCS, King Charles spaniels, Griffon Bruxel ois,
Affenpinschers, Yorkshire terriers, Maltese, Chihuahuas, Pomeranians, Boston terriers and Papil ons (52).
Chiari-like malformation has also been recognised in cross-breed dogs particularly CKCS crosses. Partly because
of its popularity as a pet, the CKCS is overrepresented and Chiari malformation is considered ubiquitous in this
breed (1, 31, 43) . Up to 65% of the Griffon Bruxel ois breed has Chiari-like malformation (21, 58); data for
other breeds is not available. Chiari-like malformation may also be seen in cats and is again more common in
brachycephalic varieties such as the Persian. The incidence of symptomatic Chiari-like malformation is not
known and is difficult to determine because the most common clinical sign is pain. Pain is a complex
amalgamation of sensation, emotions and (in humans) thoughts and manifests itself as pain behaviour (59)
which in a dog may not be recognised by owners or their veterinarians (Table 2). In addition pain associated
with Chiari-like malformation is rarely constant or focal. In humans the key features of Chiari-related headaches
are their relationship to any Valsalva-like manoeuvre, their brief duration - often lasting only seconds – and
their posterior, suboccipital location (60). In a dog this might manifest as a yelp on a rapid change of position,
for example being picked up. It is difficult to attribute non-specific and brief signs to a specific aetiology
especial y when a condition is common in a breed and can be asymptomatic. The reported number of human
patients with asymptomatic Chiari malformation type 1 varies between a third and a half of those diagnosed
with the condition by MRI (61-65).
Syringomyelia
Due to the relationship with Chiari-like malformation, prevalence of syringomyelia is also high in
brachycephalic toy-breeds (52). Again not al animals with syringomyelia are symptomatic and like Chiari-like
malformation it is difficult to obtain reliable incidence data. In humans the reported frequency of
syringomyelia in people who have Chiari malformation type 1 malformation ranges from 65 to 80% (70) and
the frequency of asymptomatic syringomyelia has been reported as being 23% (71). Syringomyelia has a
varying age of onset, there is 46% prevalence in (al egedly) asymptomatic breeding CKCS but prevalence
(symptomatic and asymptomatic) increases with age and may be as high as 70% in dogs over six years of age
(1). In the Griffon Bruxel ois 42- 52% of dogs have syringomyelia and this is not always in association with a
classical Chiari-like malformation (21, 72).
Table 2 Clinical signs of Chiari-like malformation and syringomyelia
Clinical signs
Pain Behaviour
Owners may describe spontaneous vocalisation, especial y when the dog
stands up, jumps or when it is picked up. However the expression of pain
by vocalisation is an unreliable sign and the absence of vocalisation is
not a reliable indication that the dog is comfortable
Dogs with CM with or without SM may be described as "quiet" or "lazy"
or may have decreased participation in activities such as playing and
Avoidance of rapid changes in
It is common for dogs with CM with or without SM to avoid jumping,
stairs and appear to dislike being picked up
Reduced exercise
Signs may be exacerbated by excitement and exercise, it is thought
because of increased systolic pulse pressure. Dogs with higher
neuropathic pain score have decreased wil ingness to exercise (66)].
Ear / back of skul scratching
Dogs with a wide asymmetrical
syrinx are more likely to have
phantom scratching induced by
excitement or from a non-noxious
stimulus, such as touch or wearing
a col ar (Fig 5). Scratching is
typical y unilateral and to a smal
area on the neck and /or shoulder
region. The dog does not make
skin contact (67)].

Fear / anxiety / excitability
Neuropathic pain has an important impact on an individual's quality of
life and neurobehaviour (68)]. Dogs with higher neuropathic pain scores
are more likely to have (66)]
1) Stranger-directed fear (act fearful y when approached by an
unfamiliar person).
2) Non-social fear (act fearful y when in unfamiliar situations or
when sudden loud noises occurred, e.g. thunderstorms).
3) Attachment behaviour (more ‘clingy' to the owners)
separation-related behaviour (more ‘afraid' when left alone)
4) Excitability (increased attention-seeking behaviour and more
excitable in positive, reward-associated situations)
Sleep disturbance
Dogs with higher neuropathic pain score are more likely to have
disturbed sleep (66)]. Sleeping with the head in unusual positions may be
reported (Fig 11).
Other neurological signs
Sensitivity
Dogs with symptomatic CM often
As with CM but dogs with spinal
appear to have sensitivity to
dorsal horn damage may have
palpation of the cervical and
al odynia, i.e. signs of discomfort
thoraolumbar spine.
from a non-noxious stimulus, such
as touch or grooming
Scoliosis
Dogs with a wide syrinx and dorsal
grey column damage may have
cervical torticol is and
cervicothoracic scoliosis (Fig 3).
Gait abnormalities
CKCS with CM may have subtle gait Dogs with a wide syrinx may have
abnormalities, relating to
thoracic limb weakness and muscle
cerebel ar or spinocerebel ar tract
atrophy (due to ventral horn cel
dysfunction (69)].
damage) and pelvic limb ataxia and
weakness (due to white matter
damage or involvement of the
lumbar spinal cord by the syrinx)
Exotropia
Common (related to CM)
Clinical signs
Chiari like malformation
It is recognised increasingly that Chiari-like malformation alone can cause significant morbidity and reduced
quality of life (73). As with humans with Chiari type I malformation the most important clinical sign in affected
dogs is behavioural signs of pain (Table 2). It is common for dogs with Chiari-malformation to have exotropia
(outward deviation of the eye) - typical y a ventrolateral strabismus when gazing to the ipsilateral side (Fig 2).
Figure 2 It is common for dogs
with Chiari – like malformation
to have exotropia or outward
deviation of the eye (in this case
the right eye) when gazing to the
ipsilateral side.

It is unclear whether this is oculomotor nerve/muscle palsy or related to orbit confirmation. Some human
craniosynostosis syndromes (premature fusion or abnormal development of one or more cranial sutures) with
a high prevalence of Chiari malformation (for example Apert's and Crouzon's syndrome) (22)] also have a high
prevalence of strabismus (74). Other neurological signs are detailed in Table 2. In some instances of
neurological dysfunction it is difficult to be convinced of a true association with Chiari-like malformation. For
example there is a high incidence of epilepsy in dogs with Chiari-like malformation, especial y in CKCS. In one
report, 32% of the study population had seizures (43) and in a long term study of 48 CKCS, with syringomyelia
associated neuropathic pain and where dogs with a history of seizures had been excluded from the original
cohort, 12.5 % of the study population developed epilepsy in the fol ow up period (73). Consequently it has
been suggested that there may be an association between Chiari-like malformation and epilepsy in the dog. An
association has also been suggested in humans but again it is unclear whether the association is coincidental
(75). A recent study compared ventricle size and caudal cranial fossa overcrowding in CKCS with and without
seizures and found no significant differences (48)]. Electroencephalogram evaluation, performed in three
epileptic CKCS, suggested paroxysmal abnormalities were mainly located over the frontal and temporal regions
(48). Similar changes have been reported in humans with seizures and Chiari type I malformation (76)]. Further
study is required to investigate if there is a connection between Chiari malformation and epilepsy. Vestibular
dysfunction, facial nerve paralysis and deafness may also be seen but, as with epilepsy, no direct relationship
has been proven and this association may also be circumstantial.
Figure 3 A two year old female CKCS
with cervicothoracic scoliosis and
torticollis as a consequence of
syringomyelia. The torticollis may be
confused with a head tilt associated
with vestibular dysfunction. This error
of neurological localisation may result
in a poor choice of diagnostic tests for
example performing MRI of the brain
and ears rather than the
cervicothoracic spinal cord. It is
thought that the abnormal posture is
due to asymmetrical grey matter
destruction by the expanding syrinx
resulting in an imbalance of afferent
proprioceptive information from the
cervical neuromuscular spindles
Enlarging syrinxes cause progressive neurological damage through a combination of direct pressure on neural
tissue, and ischemia. The location of functional impairment depends on the site of neuronal damage and may
include scoliosis (Fig 3), gait abnormalities and other signs, which are detailed in Table 2. However the most
important and consistent clinical sign of syringomyelia is neuropathic pain. Pain is positively correlated with
syrinx transverse width and symmetry on the vertical axis, i.e. dogs with a wider asymmetrical syrinx are more
likely to experience discomfort, and dogs with a narrow symmetrical syrinx may be asymptomatic
Figure 4 Transverse T2 weighted MRI at the level of C2
from a CKCS presenting with scratching to the right
cranial cervical region and signs of neuropathic pain.
There is an asymmetrical syrinx involving the area of the
right spinal cord dorsal horn and extending into the area
of the superficial lamina I and II.
Pain is particularly associated with asymmetrical dorsal horn involvement especialy when there is extension
into the superficial lamina I and II (Fig 4) which receive primary afferents for nociception (77) and itch (78).
Axons from projection neurons with cel bodies in lamina I cross the midline and ascend in the contralateral
white matter (for example the spinothalamic tracts) to brain stem and thalamic targets. Different types of

excitatory and inhibitory interneurons selectively innervate these projection neurons. They are also influenced
by descending serotoningergic axons originating from the raphe nuclei (77)]. It is hypothesised that disruption
to the complex synaptic circuitry in the dorsal horn is primarily responsible for the development of
neuropathic pain in syringomyelia (56, 67)].
Figure 5 "Phantom scratching" in a
CKCS. This is typically unilateral
and to the neck and shoulder region.
Here the scratching left hind limb
can be seen as a movement blur. The
dog does not make skin contact. This
action can be elicited or exacerbated
by excitement, exercise, touch and
wearing of neck collars and
harnesses. (Picture courtesy of Ms J
Harrison, Passionate Productions.)
The pathogenesis of the phantom scratching (Fig 5) is not wel understood. It has been presumed it is a
response to al odynia (discomfort or pain from a non-noxious stimulus) and / or dysaesthesia (a spontaneous
or evoked unpleasant sensation) and part of the neuropathic pain that these dogs appear to experience (56,
67)]. However it is possible that damage to inhibitory neuron circuits has permitted overexpression of a
hyperactive reflex. This may explain why mutilation is not a feature of the disease and why a minority of dogs
with phantom scratching do not appear to suffer pain. The lack of purposeful contact with the skin and the
rhythmic action is reminiscent of the "scratch reflex" described by Sherrington in 1906 (79)]. He induced this in
dogs that had undergone complete transection of the caudal cervical spinal cord. After approximately three
months, stimulation of the skin in the scapular region induced a scratching action in the ipsilateral pelvic limb.
The rhythmic action had a frequency of 4-8 times per second with the limb scratching towards but not making
contact with the skin. Like dogs with syringomyelia there was a receptive field where stimulation of the skin
induced ipsilateral pelvic limb action. Sherrington hypothesised that there was a spinal cord central pattern
generator for scratching and that this had evolved as a protective response against clinging parasites and other
irritants (79)]. It is now wel established that there are spinal cord central pattern generators for scratching
(80)]. Similar scratching action can be elicited in cats with by application of tubocurarine to the dorsal surface
of the cervical cord at C1 (and to a lesser extent C2) with the scratch being elicited by rubbing the pinna and
the skin behind the ear (81). Tubocurarine blocks Renshaw cel s, inhibitory interneurons found in the spinal
cord ventral horn (82)] that are rhythmical y active during activity such as locomotion and scratching (83)],
innervate motor neurons and receive inhibitory and excitatory synaptic inputs from commissural interneurons
and from ipsilateral locomotor networks (84)]. Hypothetical y a syrinx, particularly in the C1 / C2 region could
lead to damage to these intricate networks resulting in a scratch reflex when the appropriate dermatome is
tactilely stimulated.
Diagnosis
MRI is essential for diagnosis and determining the cause and extent of syringomyelia (Fig 1). Chiari-like
malformation is a complex disorder and although there is less phenotypic variation than with humans, there
can be differences between breeds and individuals within the same breed. In particular the conformation of
the craniocervical junction varies. A consistent feature is hindbrain and sometimes forebrain, overcrowding
with narrowing or obstruction of the CSF channels. The caudal fossa is smal and has a more horizontal y
orientated tentorium cerebel i (36, 85)]. The medul a often has a kinked appearance (85)]. The supraoccipital
bone indents the cerebel um, which loses its normal rounded shape (36, 85)]. Dilatation of the entire
ventricular system secondary to cerebrospinal fluid obstruction is common (85)]. In classical Chiari-like
malformation the cerebel um and medul a extend into or through the foramen magnum, which is occluded
with little or no CSF around the neural structures. However in some individuals the size of cerebel ar herniation
may be minimal (21)]. A flexed head position increases the size of cerebel ar herniation and is useful to
determine the extent of disease (86)]. However care is essential when obtaining these dynamic views in case
there is concomitant atlanto-axial subluxation and/or airway obstruction. The most important craniovertebral
junction abnormality associated with Chiari-like malformation is atlanto-occipital overlapping, which has been
reported as similar to basilar invagination in humans (52, 53)] (Fig 6).
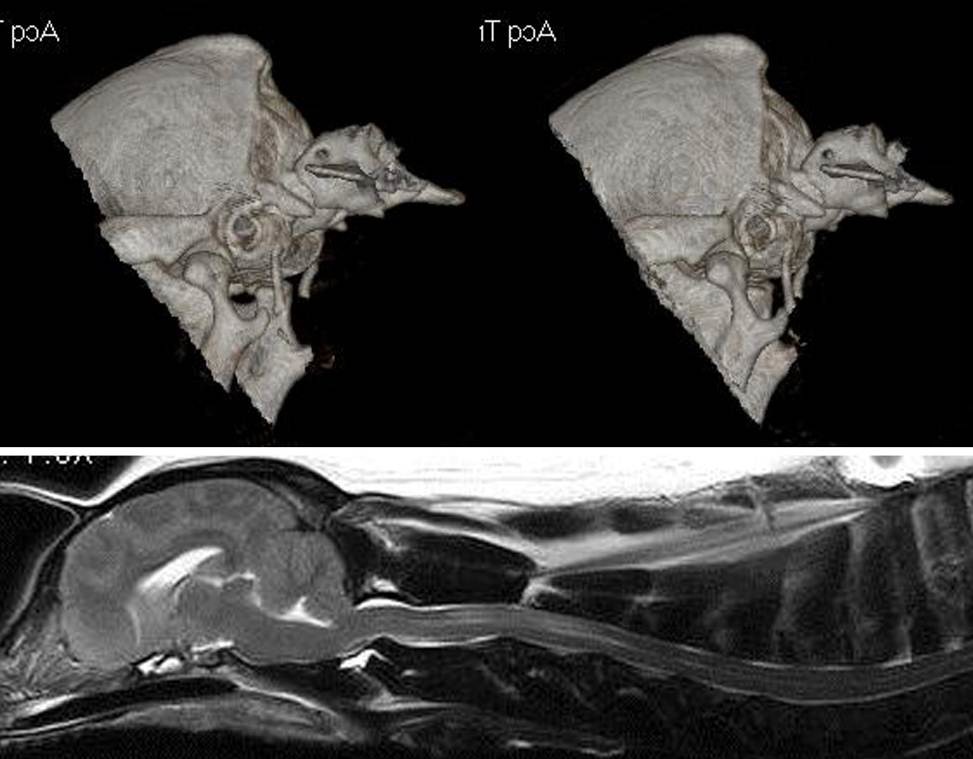
Figure 6 – Computer tomography (CT) of the caudal skul and atlas (top) and midline sagittal T2 weighted MRI of
the brain and cervical spinal cord (bottom) of a 3.5 year old male CKCS presenting with pain. The MRI reveals
Chiari-like malformation, ventriculomegaly with a mild syringomyelia and suggested atlanto-
occipital overlapping. This was confirmed by CT. It can be seen that in the extended position the atlas is over
riding the dorsal rim of the foramen magnum.
Both conditions are characterized by increased proximity of the cranial cervical spine to the base of the skul;
(87)] however, a defining characteristic of basilar invagination is displacement of the odontoid process of the
axis through the foramen magnum with compression of the medul a by the dens (87)]. In the dogs there may
be flexure of the cranial cervical spinal cord over the odontoid process but this is more subtle than the human
condition. (Fig 1) (31, 36, 52, 54)]. Other less common canine craniovertebral junction anomalies include
atlantoaxial subluxation (51, 88)] and dorsal angulation of the dens (54)]. Occipital dysplasia (i.e. widened
foramen magnum) also may be seen; (45)] however, this is probably an acquired condition due to
overcrowding of the caudal cranial fossa, mechanical pressure from the cerebel um and supraoccipital bone
resorption (89)]. It is also common to see dorsal impingement of the subarachnoid space and/or spinal cord at
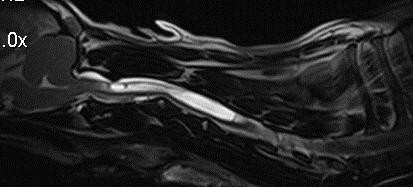
C1-C2 due to fibrosis and proliferation of the ligamentum flavum and dura (31, 36, 52)] (Fig 7).
Figure 7 Midline 3D T2-
weighted SPACE (sampling
perfection with application
optimized contrasts sequence
with different flip angle
evolutions) MRI of the caudal
skul and cervical spinal cord.
There is dorsal impingement of
the spinal cord at C1-C2. The
syringomyelia appears to start
at the level of spinal cord
impingement.
Brachycephalic dogs are also predisposed to quadrigeminal cysts (90)]. By occupying space within an already
crowded caudal cranial fossa this may aggravate the obstruction at the foramen magnum and increase the
likelihood of syringomyelia developing, although most quadrigeminal cysts are incidental findings (Fig 8).
Figure 8 Midline sagittal T1-weighted MRI images of the brain and cervical spinal cord from 1 year old female
Cavalier King Charles spaniels presenting with pain. There is a large quadrigeminal cyst in an already crowded
caudal cranial fossa. There is a large hindbrain herniation and holochord syringomyelia
Syringomyelia is indicated by fluid-containing cavities within the cervical spinal cord. When evaluating the
patient with syringomyelia then the spinal cord from C1-L4 should be imaged otherwise the extent of disease
may be underestimated (50)]. The cranial cervical and cranial thoracic segments are typical y most severely
affected. Maximum syrinx transverse width is the strongest predictor of pain, scratching behaviour and
scoliosis (56)].
Differential Diagnosis
The most important differential diagnoses are other causes of pain and spinal cord dysfunction such as
intervertebral disc disease; central nervous system inflammatory diseases such as granulomatous
meningoencephalomyelitis; vertebral abnormities such as atlantoaxial subluxation; neoplasia; and
discospondylitis. Intervertebral disc disease would be an unlikely cause of pain in a brachycephalic toy breed
aged less than 4 years old. When scratching or facial/ear rubbing is the predominant clinical sign, ear and skin
disease should be ruled out. The classic scratching behaviour for syringomyelia is to one distinct area. It is a
common incidental finding for CKCS to have a mucoid material in one or both tympanic bul ae and in the
majority of cases this is not associated with clinical signs of pain although it may cause hearing loss (43, 91)].
Some cases with scoliosis appear to have a head tilt which could be confused with vestibular dysfunction (92)]
(Fig 3). CSF analysis may be abnormal in dogs with syringomyelia possibly due to syrinx induced cel damage and
an inflammatory response in these dogs. A comparative study of CSF in CKCS with syringomyelia showed a
higher protein and cel content, as compared to those with a Chiari-like malformation and no syrinx (93)].

Figure 11
Unusual sleeping positions. Left panel CKCS with Chiari malformation and syringomyelia that routinely slept
with his head flexed and wedged behind a solid object. Picture courtesy of Ms P Persson
Right panel CKCS with Chiari malformation and syringomyelia that preferred to sleep with her hindquarters
lower than her head and with her head on a cooler surface. To achieve this, her head is on a wooden table and
her hindquarters are balanced on a cushion and the back of a sofa. ( Picture courtesy of Mrs S Smith )
Treatment
Medical and surgical treatment options exist for dogs with Chiari-like malformation with syringomyelia and a
possible approach to management is il ustrated in Fig 9. The main treatment objective is pain relief.
Surgical management
There are no clear guidelines as to when surgery is indicated over medical management because robust
outcome studies have not been performed. Some authors have suggested that early surgical intervention may
improve prognosis but this hypothesis has not been vigorously tested (94)]. The author is most likely to
recommend surgery for painful dogs with Chiari-like malformation but without marked syringomyelia and/or
dogs with syringomyelia where medical management does not give adequate pain relief. The reason why
surgery has not been recommended universal y is that no technique reported thus far has resulted in long term
syrinx resolution (94-98)]. In addition surgery does not necessarily improve long-term prognosis as 25-
47% of the operated dogs have recurrence or deterioration of the clinical signs within 0.2-3 years after surgery
(94-96)]. However, it should be remembered that it is probable that previous reports of surgical y managed
cases include dogs with more severe clinical signs so a valid comparison between medical and surgical
management cannot be made at this time.
The most common surgical management is craniocervical decompression, establishing a CSF pathway via the
removal of part of the supraoccipital bone and dorsal arch of C1 [(96, 97). Depending on the surgeon this may
be combined with a durotomy, with or without patching with a suitable graft material and with or without a
cranioplasty, using titanium mesh or other prosthesis (94, 95)]. Craniocervical decompression surgery is
successful in reducing pain and improving neurological deficits in approximately 80% of cases and
approximately 45% of cases may have a satisfactory quality of life two years postoperatively. The clinical
improvement is probably attributable to improvement in CSF flow through the foramen magnum. A
syringosubarachnoid shunting procedure using a five French equine ocular lavage catheter has also been
described. Clinical improvement in approximately 80% of cases was reported but like other reported surgeries
there was no evidence of long-term syrinx resolution on post-operative MRI and dogs stil expressed signs of
neuropathic pain post-operatively (98)].
Figure 9 – Treatment algorithm for medical management of Chiari-like malformation and syringomyelia
Medical management
Due to the persistence of syringomyelia and/or spinal cord dorsal horn damage, it is likely that the post-
operative patient wil require continuing medical management for pain relief. Also, in the majority of canine
patients, medical management alone may be chosen for financial reasons or owner preference. There are three
main type of drugs used for treatment of Chiari-like malformation with syringomyelia: drugs that reduce CSF
production (acetazolamide, cimetidine, omeprazole or furosemide); analgesics (non-steroidal anti-
inflammatory drugs and anti-epileptic drugs that have analgesic properties); and corticosteroids. As yet there
are no scientific studies to prove the efficacy of these drugs in the management of neuropathic pain in dogs
and recommended management is based on anecdotal evidence only (Fig 9).
Figure 10 It is common for dogs
with CM with or without SM to
be described as "quiet" or to
have decreased participation in
activities. This syringomyelia
affected dog's depressed
demeanour is apparent. In a
veterinary consultation room
there may be decreased
interaction with the dog
preferring to lay in sternal
recumbency with their head on
the floor
Drugs reducing cerebrospinal fluid production
The process of CSF production by the choroid plexus epithelial cel s involves the enzymes carbonic anhydrase
C, sodium and potassium ATPases, and aquaporin-1 and results in the net transport of water, sodium chloride,
potassium and bicarbonate ions from the blood into the ventricles (99)]. Acetazolamide reduces CSF
production by inhibiting carbonic anhydrase C and by reducing the amount of aquaporin-1 through an
alteration in protein transcription (100)]. The use of acetazolamide for management of Chiari-like
malformation and syringomyelia has been described (67, 85)] and is also used in management of benign
intracranial hypertension in humans (101)]. However long term use of acetazolamide is often limited by
adverse effects, including lethargy, abdominal pain and bone marrow suppression (85)].
Omeprazole is a specific inhibitor of H(+)-K(+)-activated ATPase however it is not clear if this is the mechanism
by which it reduces CSF production (102)]. In experimental models using a ventriculocisternal perfusion
technique, omeprazole reduces canine CSF production by 26% (103). Histamine (H2)-receptor antagonists such
as cimetidine and ranitidine are proposed to reduce CSF production by competitive inhibition of H2 receptors
located on the choroid plexus epithelial cel , or by a direct effect on the capil aries of the choroid plexus (104)].
However there is also evidence that histamine may act physiological y by increasing the electrical activity
of vasopressin-secreting neurons (105)]. Vasopressin reduces blood flow to the choroid plexus, thereby
decreasing CSF production (106)]. Cimetidine has been shown to be superior to ranitidine to reducing CSF
production in an experimental cat model (104)]. The usefulness of omeprazole or cimetidine for Chiari-like
malformation, with or without syringomyelia, is unclear. They are often prescribed in the hope that this may
limit disease progression, a variable that is difficult to assess in a scientific study of clinical cases. Some owners
report a significant improvement in clinical signs of pain. Adverse effects from these drugs are infrequently
reported. Cimetidine retards P450 oxidative hepatic metabolism so caution is advised if using this preparation
concurrently with other drugs metabolised by the liver and with both cimetidine and omeprazole, periodic
monitoring of haematology and serum biochemistry is advised. Absorption of gabapentin may be reduced with
concurrent cimetidine administration however the effect is thought to be clinical y insignificant (107). It has
been suggested that chronic hypergastrinemia, caused by omeprazole, may increase the risk of gastric
carcinomas, at least in laboratory rodent models but this has not been reported in any other species (108,
Use of the diuretic furosemide for management of Chiari-like malformation and syringomyelia has also been
described (67, 85)] and is also used in management of benign intracranial hypertension in humans (101)].
Furosemide may not be ideal in toy breed dogs that also have a high likelihood of mitral valve disease (110)]
and where the most common cause of death is congestive heart failure (111)]. Furosemide can result in
significant increase in plasma aldosterone concentration and renin activity in healthy dogs (112)]. This early
activation of the renin-angiotensin-aldosterone system might be deleterious in an animal predisposed to heart
disease (113)]. Moreover, long-term use of diuretics can lead to a diuretic-resistant state, which necessitates
the use of higher doses, further activating the renin-angiotensin-aldosterone system (114)].
Analgesics
NSAIDS are inhibitors of Cyclooxygenase-1 and/or Cyclooxygenase-2 and suppress inflammatory pain by
reducing generation of prostanoids, in particular prostaglandin E2. Prostaglandin E2 also contributes to the
genesis of neuropathic pain (115)]. Anecdotal y, non-steroidal anti-inflammatory drugs (NSAIDS), e.g.
meloxicam, carprofen, firocoxib, mavacoxib, can be useful in management of Chiari-like malformation and
syringomyelia. However, monotherapy with NSAIDs is unlikely to provide sufficient analgesia if there are signs
of neuropathic pain. Therefore, in these situations, the addition of drugs with an anti-al odynic effect is
recommended (67)]. All primary afferents in the spinal cord dorsal horn use glutamate as their main fast
excitatory neurotransmitter. Nociceptive afferents are divided in two groups - those that contain neuropeptide
(for example substance P and calcitonin gene related peptide and those that do not (77)].
Substance P containing primary afferents play an important part in nociception and neuropathic pain and have
a high density in laminae I and II of the spinal cord dorsal horn (77)]. Therefore drugs that affect the firing of
these neurons are useful in the management of neuropathic pain. Gabapentin and pregabalin modulate
voltage-gated calcium channels resulting in a reduction of glutamate and substance P (116)]. Anecdotal y,
pregablin is most efficacious for treating Chiari-like malformation and syringomyelia in dogs but gabapentin
can also be useful and is more economic. In severe cases that stil have clinical signs, despite polypharmacy,
the addition of opioids, tramadol or amantadine can be useful. It should be borne in mind that, with the
exception of NSAIDs, there are no licensed oral analgesics in veterinary medicine.
Corticosteroids
Corticosteroids are believed to provide long-term pain relief because of their ability to inhibit the production
of phospholipase-A-2 (117) and to inhibit the expression of multiple inflammatory genes coding for cytokines,
enzymes, receptors and adhesion molecules (118)]. Corticosteroids are also reported to reduce
sympathetical y mediated pain (119) and decrease substance P expression (120)]. Anecdotal y, oral drugs such
as methylprednisolone and prednisolone provide relief for some dogs with syringomyelia and can also be
useful where there are significant neurological deficits but adverse effects limit their usefulness for long- term
Progression and prognosis
The clinical signs of Chiari-like malformation and syringomyelia are often progressive. A long term study, over a
mean of 39±14.3 months, found that approximately three-quarters of CKCS with Chiari-like malformation and
syringomyelia associated neuropathic pain wil deteriorate when managed medical y whereas one quarter
remain static or improved (73)]. However, despite this progression, al the owners of the alive dogs in this
study reported that their dog's quality of life was not severely compromised (73)]. 15% of dogs were
euthanatised because of severe neuropathic pain. Morphometric values (volume of the caudal cranial fossa,
parenchyma within the caudal cranial fossa, and the sizes of the ventricles and syringes) were not correlated
with prognosis. Dogs with higher neuropathic pain scores are more likely to have fear-related behaviour (Table
2), which can have a negative impact on the owner-perceived quality of life of a dog (66)]. Obesity is also
positively correlated with a reduced quality of life but not greater neuropathic pain (66)]. In humans there is
also a known association between increasing body mass index and CSF disorders such as idiopathic intracranial
hypertension (121)] and syringomyelia secondary to Chiari type 1 malformation (122)]. It has not been
established if the obesity is the cause or effect of disease however in humans reducing weight can reduce syrinx
size after unsuccessful surgical decompression and reduction in body weight is recommended for al overweight
and obese patients (122)].
Figure 12 Midline sagittal T2
weighted MRI images from a 3 year
old CKCS with Chiari-like
malformation. A prominent central
canal (arrow), or early syrinx, is seen
particularly in the C2-C4 region. This
dog was not reported to have any
associated clinical signs. The MRI
was performed with a view to
Genetic factors and breeding advice
The high prevalence, within closely related populations, suggests that syringomyelia is inherited in the dog and
studies in the CKCS have shown it to be a complex trait, with a moderately high heritability (h2 = 0.37 ± 0.15
standard error) (123)]. Since the early 2000s it has been recommended that dogs of breeds predisposed to
Chiari-like malformation and/or syringomyelia be MRI screened at least twice in their lifetime. Breeding
recommendations based on syringomyelia status and ages were formulated in 2006. These guidelines
concentrated on removing dogs with early onset syringomyelia from the breeding pool whilst maintaining
genetic diversity (3)]. Early results from this breeding program indicated that offspring without syringomyelia
were more common when the parents were both clear of syringomyelia (offspring syringomyelia free; CKCS
70%, Griffon Bruxel ois 73%). Conversely offspring with syringomyelia were more likely when both parents had
syringomyelia (offspring syringomyelia affected; CKCS 92%, Griffon Bruxel ois 100%). A mating of one
syringomyelia-free parent with an syringomyelia-affected parent was risky for syringomyelia affectedness with
77% of CKCS and 46% of Griffon Bruxel ois offspring being syringomyelia affected (124)].
In the UK there is a British Veterinary Association / Kennel Club Canine Health Scheme to MRI screen potential
breeding stock for Chiari-like malformation and/or syringomyelia (125)]. MRI images are assessed by two
scrutineers and graded for severity for both Chiari-like malformation and syringomyelia and, as syringomyelia is
a late onset condition, the age of onset (Table 3). Results are submitted to a central database, in order to
generate estimated breeding values for the UK Kennel Club Mate Select Computer program (126)]. As an
accurate estimated breeding value database may take some time to compile, the recommended breeding
guidelines have been revised (127)] (Table 4). European heath schemes for Chiari-like malformation and
syringomyelia also exist (128)].
Table 3 The aim of these breeding guidelines is to remove dogs with early onset SM from the breeding
programme. Please note: it is believed that due to the complex nature of inheritance of CM/SM it is stil
possible that affected offspring may arise from parents which are clear from or are only mildly affected by SM.
British Veterinary Association (BVA) / Kennel Club (KC) CMSM Scheme
Chiari-like malformation (CM):
Grade 0 - No Chiari malformation
Grade 1 - Cerebel um indented (not rounded)
Grade 2 - Cerebel um impacted into, or herniated through, the foramen magnum.
Syringomyelia (SM)
Grade 0 - Normal (no central canal dilation, no presyrinx, no syrinx)
Grade 1 - Central canal dilation (Fig 12) or a separate syrinx, which has an internal diameter of less than 2mm
or a pre-syrinx alone.
Grade 2 - Syringomyelia (central canal dilation which has an internal diameter of 2mm or greater, a separate
syrinx, or pre-syrinx with central canal dilation).
The grade is qualified with a letter indicating the age group at the time of scanning as fol ows: a = more than
five years of age; b = three to five years of age; c = one to three years of age. The grade is not valid without
the qualifying letter.
Syringomyelia is defined as a fluid-fil ed cavity that includes or is distinct from the central canal of the spinal
cord and is graded according to its maximum internal diameter in a transverse plane.
Pre-syrinx is defined as spinal cord oedema, and may be a transitional state prior to development of
syringomyelia. Pre-syrinx has the appearance of high signal intensity on T2W images consistent with marked
increased fluid content within the spinal cord substance but not of free fluid. On T1W images the spinal cord is
either normal or has a slightly hypointense signal
Table 4 . Breeding guidelines (based on syringomyelia only)
CM – Chiari malformation, SM – syringomyelia, CCD – central canal dilatation.
No breeding guidelines for CM are available as yet. For toy breeds other than CKCS and King Charles, breeders
should aim to breed from CM1 and CM0 dogs. For breeds with almost universal CM affectedness (i.e. CKCS,
King Charles and possibly other breeds such as the Griffon Bruxel ois) then the above table below applies
Conclusion
Chiari-like malformation and syringomyelia is an inherited disorder with a high morbidity in many
brachycephalic toy breeds. It is characterised by overcrowding of the craniocervical junction, obstruction of
CSF flow through the foramen magnum and development of fluid fil ed cavities in the central spinal cord.
Although some cases are asymptomatic, dogs with Chiari-like malformation and syringomyelia can present
with neurological signs of which the most important is pain. Surgical and medical treatment options are
available but these have limited success and from a welfare point of view it would be better to implement a
breeding program limiting the occurrence of this disabling disease.
Acknowledgements
The author thanks Penny Knowler for her valued assistance in preparation of many of the figures in this paper.
Additional thanks to Taimur Alavi for his considerable help in preparation of Figure 9. Final y the author is
grateful to Colin Driver for critical y reading this manuscript and for his constructive comments.
References
Parker JE, Knowler SP, Rusbridge C, Noorman E, Jeffery ND. Prevalence of asymptomatic
syringomyelia in Cavalier King Charles spaniels. The Veterinary record. 2011;168(25):667.
Rusbridge C, Greitz D, Iskandar BJ. Syringomyelia: current concepts in pathogenesis, diagnosis, and
treatment. Journal of veterinary internal medicine / American Col ege of Veterinary Internal Medicine.
2006;20(3):469-79.
Cappel o R, Rusbridge C. Report from the Chiari-Like Malformation and Syringomyelia Working Group
round table. Veterinary surgery : VS. 2007;36(5):509-12.
Markunas CA, Tubbs RS, Moftakhar R, Ashley-Koch AE, Gregory SG, Oakes WJ, et al. Clinical,
radiological, and genetic similarities between patients with Chiari Type I and Type 0 malformations.
Journal of neurosurgery Pediatrics. 2012;9(4):372-8.
Greitz D. Unraveling the riddle of syringomyelia. Neurosurgical review. 2006;29(4):251-63; discussion
Chang HS, Nakagawa H. Hypothesis on the pathophysiology of syringomyelia based on simulation of
cerebrospinal fluid dynamics. Journal of neurology, neurosurgery, and psychiatry. 2003;74(3):344-7.
Stoodley MA, Gutschmidt B, Jones NR. Cerebrospinal fluid flow in an animal model of
noncommunicating syringomyelia. Neurosurgery. 1999;44(5):1065-75; discussion 75-6.
Stoodley MA. Pathophysiology of syringomyelia. Journal of neurosurgery. 2000;92(6):1069-70; author
Gomez DG, Potts DG. The lateral, third, and fourth ventricle choroid plexus of the dog: a structural
and ultrastructural study. Annals of neurology. 1981;10(4):333-40.
Bering EA, Jr. Choroid plexus and arterial pulsation of cerebrospinal fluid; demonstration of the
choroid plexuses as a cerebrospinal fluid pump. AMA archives of neurology and psychiatry.
1955;73(2):165-72.
Johnston M, Zakharov A, Papaiconomou C, Salmasi G, Armstrong D. Evidence of connections between
cerebrospinal fluid and nasal lymphatic vessels in humans, non-human primates and other
mammalian species. Cerebrospinal fluid research. 2004;1(1):2.
Storer KP, Toh J, Stoodley MA, Jones NR. The central canal of the human spinal cord: a computerised
3-D study. Journal of anatomy. 1998;192 ( Pt 4):565-72.
Radojicic M, Nistor G, Keirstead HS. Ascending central canal dilation and progressive ependymal
disruption in a contusion model of rodent chronic spinal cord injury. BMC neurology. 2007;7:30.
Kimelberg HK. Water homeostasis in the brain: basic concepts. Neuroscience. 2004;129(4):851-60.
Ambarki K, Baledent O, Kongolo G, Bouzerar R, Fal S, Meyer ME. A new lumped-parameter model of
cerebrospinal hydrodynamics during the cardiac cycle in healthy volunteers. IEEE transactions on bio-
medical engineering. 2007;54(3):483-91.
Heiss JD, Patronas N, DeVroom HL, Shawker T, Ennis R, Kammerer W, et al. Elucidating the
pathophysiology of syringomyelia. Journal of neurosurgery. 1999;91(4):553-62.
Wil iams B. Experimental communicating syringomyelia in dogs after cisternal kaolin injection. Part 2.
Pressure studies. Journal of the neurological sciences. 1980;48(1):109-22.
Koyanagi I, Houkin K. Pathogenesis of syringomyelia associated with Chiari type 1 malformation:
review of evidences and proposal of a new hypothesis. Neurosurgical review. 2010;33(3):271-84;
discussion 84-5.
Moritani T, Aihara T, Oguma E, Makiyama Y, Nishimoto H, Smoker WR, et al. Magnetic resonance
venography of achondroplasia: correlation of venous narrowing at the jugular foramen with
hydrocephalus. Clinical imaging. 2006;30(3):195-200.
Levine DN. The pathogenesis of syringomyelia associated with lesions at the foramen magnum: a
critical review of existing theories and proposal of a new hypothesis. Journal of the neurological
sciences. 2004;220(1-2):3-21.
Rusbridge C, Knowler SP, Pieterse L, McFadyen AK. Chiari-like malformation in the Griffon Bruxel ois.
The Journal of smal animal practice. 2009;50(8):386-93.
Cinal i G, Spennato P, Sainte-Rose C, Arnaud E, Aliberti F, Brunel e F, et al. Chiari malformation in
craniosynostosis. Child's nervous system : ChNS : official journal of the International Society for
Pediatric Neurosurgery. 2005;21(10):889-901.
Hemley SJ, Bilston LE, Cheng S, Stoodley MA. Aquaporin-4 expression and blood-spinal cord barrier
permeability in canalicular syringomyelia. Journal of neurosurgery Spine. 2012;17(6):602-12.
Bilston LE, Fletcher DF, Brodbelt AR, Stoodley MA. Arterial pulsation-driven cerebrospinal fluid flow in
the perivascular space: a computational model. Computer methods in biomechanics and biomedical
engineering. 2003;6(4):235-41.
Bilston LE, Stoodley MA, Fletcher DF. The influence of the relative timing of arterial and subarachnoid
space pulse waves on spinal perivascular cerebrospinal fluid flow as a possible factor in syrinx
development. Journal of neurosurgery. 2010;112(4):808-13.
Clarke EC, Stoodley MA, Bilston LE. Changes in temporal flow characteristics of CSF in Chiari
malformation Type I with and without syringomyelia: implications for theory of syrinx development.
Journal of neurosurgery. 2013;118(5):1135-40.
Clarke EC, Fletcher DF, Stoodley MA, Bilston LE. Computational fluid dynamics model ing of
cerebrospinal fluid pressure in Chiari malformation and syringomyelia. Journal of biomechanics. 2013.
Rennels ML, Blaumanis OR, Grady PA. Rapid solute transport throughout the brain via paravascular
fluid pathways. Advances in neurology. 1990;52:431-9.
Rennels ML, Gregory TF, Blaumanis OR, Fujimoto K, Grady PA. Evidence for a 'paravascular' fluid
circulation in the mammalian central nervous system, provided by the rapid distribution of tracer
protein throughout the brain from the subarachnoid space. Brain research. 1985;326(1):47-63.
Stoodley MA, Jones NR, Brown CJ. Evidence for rapid fluid flow from the subarachnoid space into the
spinal cord central canal in the rat. Brain research. 1996;707(2):155-64.
Cerda-Gonzalez S, Olby NJ, McCul ough S, Pease AP, Broadstone R, Osborne JA. Morphology of the
caudal fossa in Cavalier King Charles Spaniels. Veterinary radiology & ultrasound : the official journal
of the American Col ege of Veterinary Radiology and the International Veterinary Radiology
Association. 2009;50(1):37-46.
Rusbridge C, Knowler SP. Inheritance of occipital bone hypoplasia (Chiari type I malformation) in
Cavalier King Charles Spaniels. Journal of veterinary internal medicine / American Col ege of
Veterinary Internal Medicine. 2004;18(5):673-8.
Schmidt MJ, Volk H, Klingler M, Failing K, Kramer M, Ondreka N. Comparison of Closure Times for
Cranial Base Synchondroses in Mesaticephalic, Brachycephalic, and Cavalier King Charles Spaniel Dogs.
Veterinary radiology & ultrasound : the official journal of the American Col ege of Veterinary
Radiology and the International Veterinary Radiology Association. 2013.
Stockyard CR. The Genetic and Endocrinic Basis for Differences in Form and Behaviour. Anatomical
Memoirs. 19 Philadelphia: Wistar Institute of Anatomy and Biology; 1941. p. 40 - 357.
Schmidt MJ, Neumann AC, Amort KH, Failing K, Kramer M. Cephalometric measurements and
determination of general skull type of Cavalier King Charles Spaniels. Veterinary radiology &
ultrasound : the official journal of the American Col ege of Veterinary Radiology and the International
Veterinary Radiology Association. 2011;52(4):436-40.
Carrera I, Dennis R, Mel or DJ, Penderis J, Sul ivan M. Use of magnetic resonance imaging for
morphometric analysis of the caudal cranial fossa in Cavalier King Charles Spaniels. American journal
of veterinary research. 2009;70(3):340-5.
Driver CJ, Rusbridge C, Cross HR, McGonnel I, Volk HA. Relationship of brain parenchyma within the
caudal cranial fossa and ventricle size to syringomyelia in cavalier King Charles spaniels. The Journal of
smal animal practice. 2010;51(7):382-6.
Shaw TA, McGonnel IM, Driver CJ, Rusbridge C, Volk HA. Caudal cranial fossa partitioning in Cavalier
King Charles spaniels. The Veterinary record. 2013;172(13):341.
Cross HR, Cappel o R, Rusbridge C. Comparison of cerebral cranium volumes between cavalier King
Charles spaniels with Chiari-like malformation, smal breed dogs and Labradors. The Journal of smal
animal practice. 2009;50(8):399-405.
Driver CJ, Rusbridge C, McGonnel IM, Volk HA. Morphometric assessment of cranial volumes in age-
matched Cavalier King Charles spaniels with and without syringomyelia. The Veterinary record.
2010;167(25):978-9.
Fenn J, Schmidt MJ, Simpson H, Driver CJ, Volk HA. Venous sinus volume in the caudal cranial fossa in
Cavalier King Charles spaniels with syringomyelia. Vet J. 2013.
Shaw TA, McGonnel IM, Driver CJ, Rusbridge C, Volk HA. Increase in cerebel ar volume in Cavalier
King Charles Spaniels with Chiari-like malformation and its role in the development of syringomyelia.
PloS one. 2012;7(4):e33660.
Lu D, Lamb CR, Pfeiffer DU, Targett MP. Neurological signs and results of magnetic resonance imaging
in 40 cavalier King Charles spaniels with Chiari type 1-like malformations. The Veterinary record.
2003;153(9):260-3.
Driver CJ, De Risio L, Hamilton S, Rusbridge C, Dennis R, McGonnel IM, et al. Changes over time in
craniocerebral morphology and syringomyelia in cavalier King Charles spaniels with Chiari-like
malformation. BMC veterinary research. 2012;8(1):215.
Rusbridge C, Knowler SP. Coexistence of occipital dysplasia and occipital hypoplasia/syringomyelia in
the cavalier King Charles spaniel. The Journal of small animal practice. 2006;47(10):603-6.
Driver CJ, Watts V, Bunck AC, Van Ham LM, Volk HA. Assessment of cerebel ar pulsation in dogs with
and without Chiari-like malformation and syringomyelia using cardiac-gated cine magnetic resonance
imaging. Vet J. 2013.
Cerda-Gonzalez S, Olby NJ, Broadstone R, McCullough S, Osborne JA. Characteristics of cerebrospinal
fluid flow in Cavalier King Charles Spaniels analyzed using phase velocity cine magnetic resonance
imaging. Veterinary radiology & ultrasound : the official journal of the American Col ege of Veterinary
Radiology and the International Veterinary Radiology Association. 2009;50(5):467-76.
Driver CJ, Chandler K, Walmsley G, Shihab N, Volk HA. The association between Chiari-like
malformation, ventriculomegaly and seizures in cavalier King Charles spaniels. Vet J. 2012.
Schmidt MJ, Ondreka N, Rummel C, Volk H, Sauerbrey M, Kramer M. Volume reduction of the jugular
foramina in Cavalier King Charles Spaniels with syringomyelia. BMC veterinary research.
Loderstedt S, Benigni L, Chandler K, Cardwel JM, Rusbridge C, Lamb CR, et al. Distribution of
syringomyelia along the entire spinal cord in clinical y affected Cavalier King Charles Spaniels. Vet J.
Stalin CE, Rusbridge C, Granger N, Jeffery ND. Radiographic morphology of the cranial portion of the
cervical vertebral column in Cavalier King Charles Spaniels and its relationship to syringomyelia.
American journal of veterinary research. 2008;69(1):89-93.
Marino DJ, Loughin CA, Dewey CW, Marino LJ, Sackman JJ, Lesser ML, et al. Morphometric features of
the craniocervical junction region in dogs with suspected Chiari-like malformation determined by
combined use of magnetic resonance imaging and computed tomography. American journal of
veterinary research. 2012;73(1):105-11.
Cerda-Gonzalez S, Dewey CW, Scrivani PV, Kline KL. Imaging features of atlanto-occipital overlapping
in dogs. Veterinary radiology & ultrasound : the official journal of the American Col ege of Veterinary
Radiology and the International Veterinary Radiology Association. 2009;50(3):264-8.
Bynevelt M, Rusbridge C, Britton J. Dorsal dens angulation and a Chiari type malformation in a
Cavalier King Charles Spaniel. Veterinary radiology & ultrasound : the official journal of the American
Col ege of Veterinary Radiology and the International Veterinary Radiology Association.
2000;41(6):521-4.
Carruthers H, Rusbridge C, Dube MP, Holmes M, Jeffery N. Association between cervical and
intracranial dimensions and syringomyelia in the cavalier King Charles spaniel. The Journal of smal
animal practice. 2009;50(8):394-8.
Rusbridge C, Carruthers H, Dube MP, Holmes M, Jeffery ND. Syringomyelia in cavalier King Charles
spaniels: the relationship between syrinx dimensions and pain. The Journal of smal animal practice.
2007;48(8):432-6.
Hu HZ, Rusbridge C, Constantino-Casas F, Jeffery N. Histopathological investigation of syringomyelia in
the Cavalier King Charles Spaniel. Journal of comparative pathology. 2012;146(2):192-201.
Freedman D. Preliminary Morphometric Evaluation of Syringomyelia in American Brussels Griffon
Dogs. Journal of Veterinary Internal Medicine. 2011;25(3).
Tripp DA, Nickel JC. Psychosocial Aspects of Chronic Pelvic Pain International Association for the Study
of Pain: Pain Clinical Updates 2013;XXI(1):1-7.
Muel er DM, Oro JJ. Prospective analysis of presenting symptoms among 265 patients with
radiographic evidence of Chiari malformation type I with or without syringomyelia. Journal of the
American Academy of Nurse Practitioners. 2004;16(3):134-8.
Benglis D, Jr., Covington D, Bhatia R, Bhatia S, Elhammady MS, Ragheb J, et al. Outcomes in pediatric
patients with Chiari malformation Type I followed up without surgery. Journal of neurosurgery
Pediatrics. 2011;7(4):375-9.
Elster AD, Chen MY. Chiari I malformations: clinical and radiologic reappraisal. Radiology.
1992;183(2):347-53.
Meadows J, Kraut M, Guarnieri M, Haroun RI, Carson BS. Asymptomatic Chiari Type I malformations
identified on magnetic resonance imaging. Journal of neurosurgery. 2000;92(6):920-6.
Novegno F, Caldarel i M, Massa A, Chieffo D, Massimi L, Pettorini B, et al. The natural history of the
Chiari Type I anomaly. Journal of neurosurgery Pediatrics. 2008;2(3):179-87.
Wu YW, Chin CT, Chan KM, Barkovich AJ, Ferriero DM. Pediatric Chiari I malformations: do clinical and
radiologic features correlate? Neurology. 1999;53(6):1271-6.
Rutherford L, Wessmann A, Rusbridge C, McGonnel IM, Abeyesinghe S, Burn C, et al. Questionnaire-
based behaviour analysis of Cavalier King Charles spaniels with neuropathic pain due to Chiari-like
malformation and syringomyelia. Vet J. 2012.
Rusbridge C, Jeffery ND. Pathophysiology and treatment of neuropathic pain associated with
syringomyelia. Vet J. 2008;175(2):164-72.
Gustorff B, Dorner T, Likar R, Grisold W, Lawrence K, Schwarz F, et al. Prevalence of self-reported
neuropathic pain and impact on quality of life: a prospective representative survey. Acta
anaesthesiologica Scandinavica. 2008;52(1):132-6.
Suiter EJ, E. O, Pfau T, Volk HA, editors. Objective Quantification of Gait Deficits in Cavalier King
Charles Spaniels with Chiari-Like Malformation and Syringomyelia. 25th Annual Symposium of ESVN
and ECVN; 2012; Ghent
Speer MC, George TM, Enterline DS, Franklin A, Wolpert CM, Milhorat TH. A genetic hypothesis for
Chiari I malformation with or without syringomyelia. Neurosurgical focus. 2000;8(3):E12.
Sakushima K, Tsuboi S, Yabe I, Hida K, Terae S, Uehara R, et al. Nationwide survey on the epidemiology
of syringomyelia in Japan. Journal of the neurological sciences. 2012;313(1-2):147-52.
Knowler SP, McFadyen AK, Rusbridge C. Effectiveness of breeding guidelines for reducing the
prevalence of syringomyelia. Veterinary Record. 2011;169(26):681-.
Plessas IN, Rusbridge C, Driver CJ, Chandler KE, Craig A, McGonnel IM, et al. Long-term outcome of
Cavalier King Charles spaniel dogs with clinical signs associated with Chiari-like malformation and
syringomyelia. The Veterinary record. 2012.
Lehman S. Strabismus in craniosynostosis. Current opinion in ophthalmology. 2006;17(5):432-4.
Granata T, Valentini LG. Epilepsy in type 1 Chiari malformation. Neurological sciences : official journal
of the Italian Neurological Society and of the Italian Society of Clinical Neurophysiology. 2011;32 Suppl
Elia M, Biondi R, Sofia V, Musumeci SA, Ferri R, Capovil a G, et al. Seizures in Chiari I malformation: a
clinical and electroencephalographic study. Journal of child neurology. 1999;14(7):446-50.
Todd AJ, editor. Neuronal circuits and receptors involved in spinal cord pain processing Seattle: ISAP
Ross SE, Mardinly AR, McCord AE, Zurawski J, Cohen S, Jung C, et al. Loss of inhibitory interneurons in
the dorsal spinal cord and elevated itch in Bhlhb5 mutant mice. Neuron. 2010;65(6):886-98.
Sherrington CS. Observations on the scratch-reflex in the spinal dog. The Journal of physiology.
1906;34(1-2):1-50.
Frigon A. Central pattern generators of the mammalian spinal cord. The Neuroscientist : a review
journal bringing neurobiology, neurology and psychiatry. 2012;18(1):56-69.
Domer FR, Feldberg W. Scratching movements and facilitation of the scratch reflex produced by
tubocurarine in cats. The Journal of physiology. 1960;153:35-51.
al-Zamil Z, Bagust J, Kerkut GA. Tubocurarine and strychnine block Renshaw cel inhibition in the
isolated mammalian spinal cord. General pharmacology. 1990;21(4):499-509.
Deliagina TG, Fel'dman AG. [Modulation of Renshaw cel activity during scratching]. Neirofiziologiia =
Neurophysiology. 1978;10(2):210-1.
Nishimaru H, Restrepo CE, Kiehn O. Activity of Renshaw cells during locomotor-like rhythmic activity
in the isolated spinal cord of neonatal mice. The Journal of neuroscience : the official journal of the
Society for Neuroscience. 2006;26(20):5320-8.
Rusbridge C, MacSweeny JE, Davies JV, Chandler K, Fitzmaurice SN, Dennis R, et al.
Syringohydromyelia in Cavalier King Charles spaniels. Journal of the American Animal Hospital
Association. 2000;36(1):34-41.
Upchurch JJ, McGonnel IM, Driver CJ, Butler L, Volk HA. Influence of head positioning on the
assessment of Chiari-like malformation in Cavalier King Charles spaniels. The Veterinary record.
2011;169(11):277.
Botelho RV, Ferreira ED. Angular craniometry in craniocervical junction malformation. Neurosurgical
Rusbridge C. Neurological diseases of the Cavalier King Charles spaniel. The Journal of smal animal
practice. 2005;46(6):265-72.
Driver CJ, De Risio L, Hamilton S, Rusbridge C, Dennis R, McGonnel IM, et al. Changes over time in
craniocerebral morphology and syringomyelia in cavalier King Charles spaniels with Chiari-like
malformation. BMC veterinary research. 2012;8(1):1-7.
Matiasek LA, Platt SR, Shaw S, Dennis R. Clinical and magnetic resonance imaging characteristics of
quadrigeminal cysts in dogs. Journal of veterinary internal medicine / American Col ege of Veterinary
Internal Medicine. 2007;21(5):1021-6.
Harcourt-Brown TR, Parker JE, Granger N, Jeffery ND. Effect of middle ear effusion on the brain-stem
auditory evoked response of Cavalier King Charles Spaniels. Vet J. 2011;188(3):341-5.
Rusbridge C. Neurological diseases of the Cavalier King Charles spaniel. Journal of Smal Animal
Practice. 2005;46(6):265-72.
Whittaker DE, English K, McGonnel IM, Volk HA. Evaluation of cerebrospinal fluid in Cavalier King
Charles Spaniel dogs diagnosed with Chiari-like malformation with or without concurrent
syringomyelia. Journal of veterinary diagnostic investigation : official publication of the American
Association of Veterinary Laboratory Diagnosticians, Inc. 2011;23(2):302-7.
Dewey CW, Berg JM, Barone G, Marino DJ, Stefanacci JD. Foramen magnum decompression for
treatment of caudal occipital malformation syndrome in dogs. Journal of the American Veterinary
Medical Association. 2005;227(8):1270-5, 50-1.
Dewey CW, Marino DJ, Bailey KS, Loughin CA, Barone G, Bolognese P, et al. Foramen magnum
decompression with cranioplasty for treatment of caudal occipital malformation syndrome in dogs.
Veterinary surgery : VS. 2007;36(5):406-15.
Rusbridge C. Chiari-like malformation with syringomyelia in the Cavalier King Charles spaniel: long-
term outcome after surgical management. Veterinary surgery : VS. 2007;36(5):396-405.
Vermeersch K, Van Ham L, Caemaert J, Tshamala M, Taeymans O, Bhatti S, et al. Suboccipital
craniectomy, dorsal laminectomy of C1, durotomy and dural graft placement as a treatment for
syringohydromyelia with cerebel ar tonsil herniation in Cavalier King Charles spaniels. Veterinary
surgery : VS. 2004;33(4):355-60.
Motta L, Skerritt GC. Syringosubarachnoid shunt as a management for syringohydromyelia in dogs.
The Journal of smal animal practice. 2012;53(4):205-12.
Brown PD, Davies SL, Speake T, Mil ar ID. Molecular mechanisms of cerebrospinal fluid production.
Neuroscience. 2004;129(4):957-70.
Ameli PA, Madan M, Chigurupati S, Yu A, Chan SL, Pattisapu JV. Effect of acetazolamide on aquaporin-
1 and fluid flow in cultured choroid plexus. Acta Neurochir Suppl. 2012;113:59-64.
Phil ips PH. Pediatric pseudotumor cerebri. International ophthalmology clinics. 2012;52(3):51-9, xi .
Lindval -Axelsson M, Nilsson C, Owman C, Winbladh B. Inhibition of cerebrospinal fluid formation by
omeprazole. Experimental neurology. 1992;115(3):394-9.
Javaheri S, Corbett WS, Simbartl LA, Mehta S, Khosla A. Different effects of omeprazole and Sch 28080
on canine cerebrospinal fluid production. Brain research. 1997;754(1-2):321-4.
Naveh Y, Kitzes R, Lemberger A, Ben-David S, Feinsod M. Effect of histamine H2 receptor antagonists
on the secretion of cerebrospinal fluid in the cat. Journal of neurochemistry. 1992;58(4):1347-52.
Armstrong WE, Sladek CD. Evidence for excitatory actions of histamine on supraoptic neurons in vitro:
mediation by an H1-type receptor. Neuroscience. 1985;16(2):307-22.
Faraci FM, Mayhan WG, Heistad DD. Effect of vasopressin on production of cerebrospinal fluid:
possible role of vasopressin (V1)-receptors. The American journal of physiology. 1990;258(1 Pt 2):R94- 8.
National-Library-of-Medicine. GABAPENTIN solution
U.S. National Library of Medicine; 2013 [updated March 2013; cited 2013 8th July ].
Hagiwara T, Mukaisho K, Nakayama T, Sugihara H, Hattori T. Long-term proton pump inhibitor
administration worsens atrophic corpus gastritis and promotes adenocarcinoma development in
Mongolian gerbils infected with Helicobacter pylori. Gut. 2011;60(5):624-30.
Chapman DB, Rees CJ, Lippert D, Sataloff RT, Wright SC, Jr. Adverse effects of long-term proton pump
inhibitor use: a review for the otolaryngologist. Journal of voice : official journal of the Voice
Foundation. 2011;25(2):236-40.
Lewis T, Swift S, Wool iams JA, Blott S. Heritability of premature mitral valve disease in Cavalier King
Charles spaniels. Vet J. 2011;188(1):73-6.
Adams VJ, Evans KM, Sampson J, Wood JL. Methods and mortality results of a health survey of
purebred dogs in the UK. The Journal of smal animal practice. 2010;51(10):512-24.
Pedersen HD. Effects of mild mitral valve insufficiency, sodium intake, and place of blood sampling on
the renin-angiotensin system in dogs. Acta veterinaria Scandinavica. 1996;37(1):109-18.
Connel JM, MacKenzie SM, Freel EM, Fraser R, Davies E. A lifetime of aldosterone excess: long-term
consequences of altered regulation of aldosterone production for cardiovascular function. Endocrine
reviews. 2008;29(2):133-54.
Parrinel o G, Torres D, Paterna S. Salt and water imbalance in chronic heart failure. Internal and
emergency medicine. 2011;6 Suppl 1:29-36.
Kawabata A. Prostaglandin E2 and pain--an update. Biological & pharmaceutical bul etin.
2011;34(8):1170-3.
Tremont-Lukats IW, Megeff C, Backonja MM. Anticonvulsants for neuropathic pain syndromes:
mechanisms of action and place in therapy. Drugs. 2000;60(5):1029-52.
Nolan AM. Pharmacology of Analgesic drugs. In: Flecknel PA, Waterman-Pearson A, editors. Pain
Management in Animals. London: W.B. Saunders; 2000. p. 21-52.
Barnes PJ. Anti-inflammatory actions of glucocorticoids: molecular mechanisms. Clin Sci (Lond).
1998;94(6):557-72.
Gel man H. Reflex sympathetic dystrophy: alternative modalities for pain management. Instructional
course lectures. 2000;49:549-57.
Wong HK, Tan KJ. Effects of corticosteroids on nerve root recovery after spinal nerve root
compression. Clinical orthopaedics and related research. 2002(403):248-52.
Hannerz J, Ericson K. The relationship between idiopathic intracranial hypertension and obesity.
Headache. 2009;49(2):178-84.
Arnautovic KI, Muzevic D, Splavski B, Boop FA. Association of increased body mass index with Chiari
malformation Type I and syrinx formation in adults. Journal of neurosurgery. 2013.
Lewis T, Rusbridge C, Knowler P, Blott S, Wool iams JA. Heritability of syringomyelia in Cavalier King
Charles spaniels. Vet J. 2010;183(3):345-7.
Knowler SP, McFadyen AK, Rusbridge C. Effectiveness of breeding guidelines for reducing the
prevalence of syringomyelia. Veterinary Record. 2011.
BVA T. Chiari Malformation/Syringomyelia Scheme (CM/SM Scheme)
VA, The; 2013 [cited 2013 8th July ].
Club TK. Mate Select Online Service
lub, The Kennel; 2012 [cited 2013 8th July
BVA T. Appendix 1 Breeding recommendations until relevant EBVs are available [PDF].
[cited 2013 8th July ].
Jacques A. Cavaliers for Lif
[cited 2013 8th July].
Schmidt MJ, Roth J, Ondreka N, Kramer M, Rummel C. A potential role for substance P and
interleukin-6 in the cerebrospinal fluid of Cavalier King Charles Spaniels with neuropathic pain. Journal
of veterinary internal medicine / American Col ege of Veterinary Internal Medicine. 2013;27(3):530-5.
Van Biervliet J, de Lahunta A, Ennulat D, Oglesbee M, Summers B. Acquired cervical scoliosis in six
horses associated with dorsal grey column chronic myelitis. Equine veterinary journal. 2004;36(1):86- 92.
Source: http://www.veterinary-neurologist.co.uk/resources/EUROPEAN-J-COMP-AN-PRA-20_7.pdf
Microsoft word - blanqueamiento.doc
Blanqueamiento de dientes con decoloraciones severas Dr. Salvador Alonso Pérez Prof. Colaborador de Materiales Odontológicos. Facultad de Odontología Universidad de Barcelona. Doctor en Medicina. Médico Especialista en Estomatología y en Cirugía Dra. Soledad Espías Gómez Prof. Colaborador de Materiales Odontológicos. Facultad de Odontología Universidad de
A greener approach to aspirin synthesis using microwave irradiation
In the Laboratory Mary M. Kirchhoff ACS Green Chemistry Institute Washington, DC 20036 A Greener Approach to Aspirin SynthesisUsing Microwave Irradiation Ingrid Montes,* David Sanabria, Marilyn García, Joaudimir Castro, and Johanna FajardoDepartment of Chemistry, University of Puerto Rico, San Juan, Puerto Rico 00931-3349; *[email protected]