Ghaffarietal2014.pdf
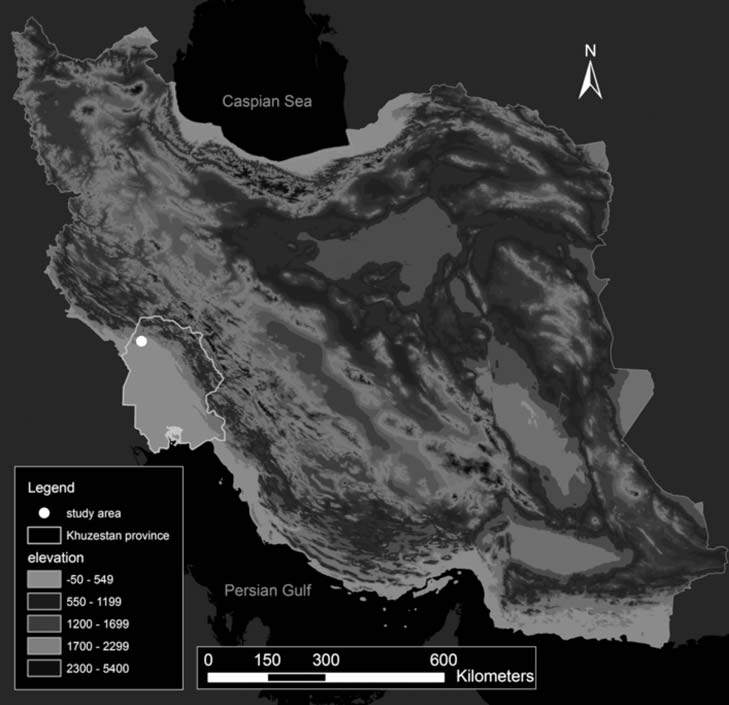
Chelonian Conservation and Biology, 2014, 13(2): 202–215 g 2014 Chelonian Research Foundation Home Range and Habitat Selection of the Endangered Euphrates Softshell Turtle Rafetus euphraticus in a Fragmented Habitat in Southwestern Iran *, FLORA IHLOW , MICHAEL V. PLUMMER , MAHMOOD KARAMI , EMATOLLAH KHORASANI , BARBOD SAFAEI-MAHROO , AND DENNIS RO 1Department of Environmental Science, Graduate School of the Environment and Energy, Science and Research Branch, Islamic Azad University, Tehran, Iran [[email protected]; [email protected]; [email protected]; [email protected]]; 2Herpetological Department, Zoologisches Forschungsmuseum Alexander Koenig (ZFMK), Adenauerallee 160, 53113, Bonn, Germany 3Department of Biology, Harding University, Searcy, Arkansas 72149 USA [[email protected]]; 4The first two authors contributed equally to this article *Corresponding author ABSTRACT. – We present information on movement patterns and habitat selection of theendangered Euphrates softshell turtle Rafetus euphraticus (Daudin 1802) from KarkhehRegulating Dam Lake in southwestern Iran. Twelve adult turtles were trapped, fitted withradio-tracking transmitters, and relocated 21 to 51 times between May 2011 and July 2012. Themean linear range size was 2.54 ± 0.83 km, the mean river channel area was 55.35 ± 17.98 ha,the mean minimum convex polygon (MCP) size was 47.49 ± 23.36 ha, and the mean 95% kerneldensity estimator (KDE 95%) measured 21.75 ± 9.44 ha with a core area (KDE 50%) of5.74 ± 2.87 ha. Range overlap was generally high; on average, individual MCPs overlapped withthose of 7.5 other turtles, individual KDEs with those of 7.3 other turtles, and core areas withthose of 5.5 other turtles. Selection of habitat types was not proportional to availability. Studyanimals preferred shallow-water edge habitats covered with Phragmites australis over all otherhabitat types.KEY WORDS. – habitat selection; fixed kernel density estimator; minimum convex polygon; linearhome range; radio-tracking; Khuzestan Province The Euphrates softshell turtle, Rafetus euphraticus the species' habitat requirements and movement ecology (Daudin 1802), is a highly aquatic and cryptic trionychid (Pittman and Dorcas 2009). So far the species has been turtle found in the Euphrates and Tigris rivers and their studied almost exclusively in Turkey (Gramentz 1991; tributaries in Turkey, Syria, Iraq, and Iran (Tas¸kavak and Tas¸kavak and Atatu¨r 1995; Biricik and Turg˘a 2011). The Atatu¨r 1995, 1998; Ghaffari et al. 2008; Biricik and Turg˘a present study reports the first data on movement patterns, 2011). In Iran, the species is restricted to the Karoon, home range sizes, habitat selection, and basking of the Karkheh, Dez, and Jarahi rivers and their tributaries as well endangered species from a fragmented habitat in south- as the Hawr-al-Azim marshlands in the southwestern part western Iran.
of the country (Ghaffari et al. 2008). Throughout its rangeR. euphraticus is severely threatened by ongoing habitat destruction and fragmentation caused by conflicts and warsin the past, by drainage to reclaim areas for agricultural Study Area. — The Karkheh Regulating Dam Lake purposes, and by an increasing number of dams (Tas¸kavak (KRDL) is situated in the northwestern part of Khuzestan and Atatu¨r 1995; Partow 2001; Ihlow et al. 2014). The Province in southwestern Iran (Fig. 1). It was part of the species is also affected by water pollution through Karkheh River until the construction of the Pay-e-Pol fertilizers and pesticides, oil, garbage, industrial chemicals, Regulating and Diversion Dam, which separated it from and incidental capture with fishing gear (Ghaffari et al.
the main river in 2009. The study area is bordered by the 2008). Populations have been reported to be declining in Karkheh Dam in the north and by the Pay-e-Pol Turkey and Iran (Gramentz 1991; Tas¸kavak and Atatu¨r Regulating and Diversion Dam in the south. The 1995; Ghaffari et al. 2008; Biricik and Turg˘a 2011). In meandering lake measures 266.42 ha, is 101 to 658 m 1996, R. euphraticus was consequently listed as endan- wide, and stretches10 km from north to south. The lake is gered on the International Union for Conservation of generally deep (10–15 m) but also has shallow edges and Nature (IUCN) Red List of Threatened Species (Biricik several small islands. Tributaries and channels range and Turg˘a 2011; IUCN 2013).
from 30 to 67 m in width. The KRDL is spring-fed by Impact assessments regarding habitat loss and drivers numerous natural springs. The water level is regulated by for population decline are currently lacking and are dam gates and is highly variable. During the summer and difficult to formulate without appropriate knowledge on autumn months, a few small temporary ditches exist in
GHAFFARI ET AL. — Home Range of Euphrates Softshell Turtle in Iran
Figure 1. Topographic map of Iran, displaying the study area in Khuzestan Province as a black dot. Map designed using ArcGis 9.3.
Elevation data: CGIAR SRTM (Jarvis et al. 2008).
close proximity to the northern edge of the lake that
Radiotelemetry and Data Collection. — Fourteen R.
potentially serve as nurseries for R. euphraticus hatch-
euphraticus were caught in a large submerged turtle trap.
lings. The rich submerged vegetation includes Potamo-
The trap design was developed based on local fishermen's
geton pectinatus and Ceratophyllum demersum. The lake
experience and constructed of iron bars and chicken wire
is partly encompassed by a dense stand of Phragmites
(Fig. 4). It was baited with approximately 400 g of fresh
australis, which reaches 3 m in height. The surrounding
chicken intestines placed in bags made of chicken wire.
area is mainly covered by shrubs including Tamarix spp.
Empty water-bottle buoys marked trap locations and
and Prosopis farcta and a few scattered trees, mainly
facilitated retrieval. Although trapping in shallow water
Populus euphratica and Ziziphus spina-christi (Figs. 2
was more successful in previous studies in Turkey
and 3). Several stretches of shoreline without any
(Gramentz 1991), the trap was placed in a depth of 10 m
vegetation potentially serve as basking or nesting sites.
to prevent it from being taken by local fishermen. The
Human population density is generally low, but the
trap was checked every 8–12 hrs to prevent captured
area is frequently used by local people for fishing,
turtles from drowning (Kuchling 2003). Although R.
boating, hunting, and camping. Vertebrate species found
euphraticus was reported to be mostly diurnal (Gramentz
in the KRDL include Caspian pond turtles (Mauremys
1991; Tas¸kavak and Atatu¨r 1995), trapping was unsuc-
caspica siebenrocki), various species of fish (including
cessful during the daytime (between 1000 and 1700 hrs,
several species of the genus Barbus, Cyprinus carpio,
n 5 6 d). Thus, trapping was performed during the night
Cyprinion macrostomum, Glyptothorax kurdistanicus, and
between 2000 and 0800 hrs (n 5 13 nights). The trap was
Glyptothorax silviae), and several species of amphibians
placed in 13 different locations between 1 April and 31
(e.g., Pseudepidalea variabilis, Hyla savignyi, and
May 2011 (Fig. 4). Fourteen R. euphraticus were caught,
Pelophylax ridibundus). Numerous invertebrates, includ-
including 2 juveniles with straight-line carapace lengths
ing abundant insect larvae, aquatic insects, and snail
(SCL) , 15 cm and 12 turtles with body sizes suitable for
species, serve as potential prey for Rafetus.
radio tracking (SCL . 29 cm).
CHELONIAN CONSERVATION AND BIOLOGY, Volume 13, Number 2 – 2014
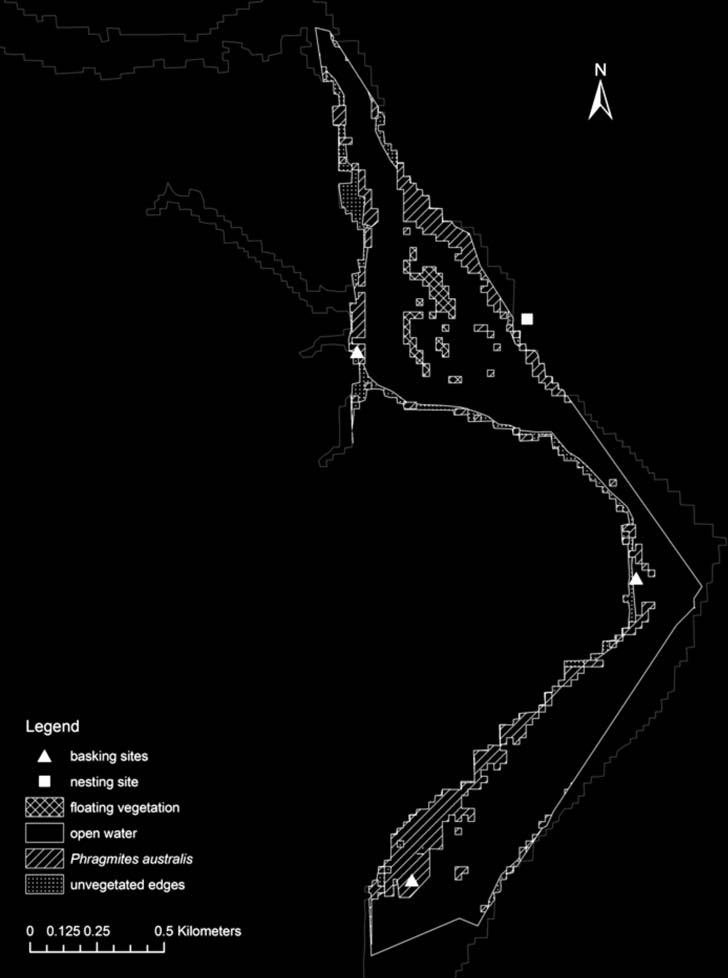
Figure 2. Map of the Karkheh Regulating Dam Lake highlighting the 4 major habitat types available at the study site.
Captured turtles were marked for individual identi-
diameter) through 2 holes punched with a needle in the
fication using a notching system modified for softshell
posterior margin of the turtles' carapace (Fig. 5). The
turtles (Plummer 2008). Morphometric characteristics of
wire was passed through a plastic button on the ventral
turtles were collected following Tas¸kavak and Atatu¨r
plastral surface to prevent the transmitter from pulling
(1998) using digital calipers (202010, Vogel Germany
out. The mean weight of the transmitter assembly totaled
GmbH & Co. KG, Kevelaer, Germany). Measurements
35 g and therefore was less than 1.1% of the smallest
were taken to the nearest 0.01 mm. The 12 turtles
turtle's body mass (BM; Table 1) and well below the 10%
exceeding 29 cm SCL were taken to the Department of
recommended maximum for reptiles (Anonymous 1987).
Environment in Dezful and fitted with radio-tracking
All turtles tagged were released at their capture locations
transmitters (164 MHz; Al-2F, Holohil Systems Ltd.,
within 2 d of capture.
Caro Ontario, Canada) by professional veterinarians.
Fieldwork was carried out for 1 wk per month
After testing, transmitters were mounted on aluminum
between May and October 2011, 2 d in January and
plates and attached with stainless steel wire (0.9-mm
March 2012, and for 1 wk per month between April and

GHAFFARI ET AL. — Home Range of Euphrates Softshell Turtle in Iran
Figure 3. Habitat of R. euphraticus at the Karkheh Regulating Dam Lake in Khuzestan Province, Iran. Left: shallow-water shorelinescovered with Phragmites australis. Right: calm open water. Photographs by Hanyeh Ghaffari.
July 2012. During fieldwork, turtles were tracked daily
Blouin-Demers 2006; Ryan et al. 2006). Furthermore,
between 0800 and 1800 hrs by boat using a hand-held
MCPs often include unused or unavailable habitats such
receiver (TRX-1000S W, 164 MHZ, Wildlife Materials
as terrestrial habitats for highly aquatic species. To
International Inc., Illinois, USA) and a 3 element fold-
address this issue the terrestrial portion of each MCP
ing Yagi antenna (Yagi 3 Element Folding Antenna,
was excluded based on satellite pictures (Indian Remote
164 MHZ, Wildlife Materials, Inc., Murphysboro, IL).
Sensing satellite map, resolution 24 m). An MCP is usually
Locations were recorded using a hand-held global
dependent on the number of fixes (Jenrich and Turner
positioning system unit (GPS map 78s, Garmin Interna-
1969). Due to several field constraints, equal numbers
tional Inc., Olathe, KS). At the end of the tracking study
of fixes could not be gathered for turtles. Despite the
all radio-tracking transmitters were carefully removed
disadvantages of the MCP method, it is the most frequently
from the turtles' shells.
used approach to analyze animal movement (Powell 2000;
Habitat Selection. — Based on remote sensing data
Nilson et al. 2008) and therefore can facilitate comparisons
(Indian Remote Sensing satellite image, 2007), we
of results with previous studies (Nilson et al. 2008). MCPs
constructed a habitat map that subdivided the study area
were calculated using ArcGis 9.3 and the Hawth's Analysis
into 4 major habitat types to which turtle locations were
Tool extension (Beyer 2004).
assigned (Fig. 2): 1) shallow-water shorelines covered by
The KDE provides a probability range around each
Phragmites australis (20.22 ha, 17%); 2) shallow-water
location, giving areas used more frequently a higher
shorelines without any vegetation (7.3 ha, 6%); 3)
value; it therefore provides information on habitat
floating vegetation and shallow vegetated areas inside
selection patterns by quantifying the intensity of use
the KRDL (2.89 ha, 2%); and 4) open, deep water
within an area (Row and Blouin-Demers 2006). Estimates
(85.59 ha, 74%).
of total home range (95% KDEs) and core areas (50%
Data Analysis. — ArcGis 9.3 (ESRI, Redlands, CA)
KDEs) were performed using ESRI ArcGis 9.3 and the
was used to measure linear range (LR) size as the straight-
Hawth's Analysis Tool extension. The smoothing param-
line distance between the most distant locations of each
eter h was determined by least-square cross validation
turtle (Sexton 1959; Pluto and Bellis 1988; Lue and Chen
using Animal Space Use 1.3 (Horne and Garton 2009). To
1999). Because the species is highly aquatic, LRs crossing
ensure comparability of KDEs, the mean smoothing
terrestrial areas were modified to represent the shortest
parameter (h 5 50.22) was used as recommended by
distance in water (Carrie re 2007).
Kenward (2001). Due to the turtles' highly aquatic
Turtles' movements were analyzed using a river
lifestyle, the terrestrial portion was excluded from the
channel area (RCA) estimator, a minimum convex polygon
resulting KDEs based on satellite pictures. In addition,
estimator (100% MCP; Mohr 1947), and 95% and 50%
interindividual overlap areas of MCPs, KDEs, and core
fixed kernel density estimators (KDE). The RCA was
areas were compared. One individual was excluded from
determined by multiplying the aquatic LR length of each
the analysis due to an insufficient number of fixes (n 5 6;
turtle by average river width (Plummer et al. 1997; Doody
minimum number of fixes required 5 20).
et al. 2002; Kay 2004; Souza et al. 2008).
The term ‘‘home range'' was applied as defined by
The MCP connects the outermost relocation points,
Kenward (2001) as ‘‘an area repeatedly traversed by
which yields a convex polygon that provides a maximum
the study animal.'' An incremental area analysis was
home range estimate but does not provide information on
performed on MCP estimates of home range to assess
habitat use and selection (Kenward 2001; Row and
whether home range size estimates reached asymptotes,
CHELONIAN CONSERVATION AND BIOLOGY, Volume 13, Number 2 – 2014
1998). A total of 100 RWMs were generated for eachturtle's kernel density estimate home range and core areaand compared with real observed movement. Animalsare deemed to be exhibiting site fidelity when observeddistributions of real individuals are significantly smallerthan computer-simulated RWMs (Munger 1984; Spenceret al. 1990).
Range overlap for each turtle was determined as the
percentage of its total home range that overlapped rangesof other turtles (Geffen and Mendelssohn 1988). Theanalysis was performed using the ‘‘adehabitat'' packagefor Cran R (Calenge 2006).
Data were checked for normality using a Kolmogorov-
Smirnov test and log10-transformed prior to statisticalanalysis with SPSS 14.0 (SPSS Inc., Chicago, IL).
Significance was determined at a 5 0.05. The relationshipof range size and body size was determined using aSpearman's rank correlation test. Potential habitat selectionwas determined using a x2 goodness-of-fit test (Neu et al.
1974; Manly et al. 2002; Ryan et al. 2006). Confidenceintervals were determined using a Bonferroni z-test (Neuet al. 1974; Ryan et al. 2006). Range estimates tend toincrease with number of fixes, which may lead to a bias ifsample sizes obtained are variable among study animals(White and Garrott 1990). As sample size in this study washighly variable, a linear regression analysis of MCP sizeson number of fixes was performed to analyze the data setfor a potential bias due to sample size (Dreslik et al. 2003).
Means are reported as ± 1 standard deviation (SD).
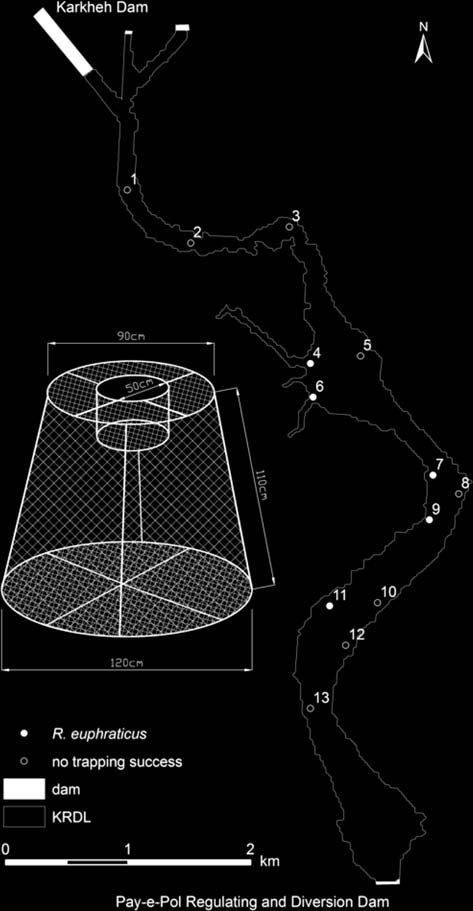
Turtle Trapping. — Trapping was successful in 5 of
13 trapping locations (38.5%), all along the western shore(Fig. 4). The highest numbers of R. euphraticus werecaught in locations 6 and 9 (n 5 3 each). Successfultrapping locations were less than 20 m from denselyvegetated water edges (habitat type 1). Two successfultrapping locations (4 and 6) were situated at the entrance
Figure 4. Industrial drawing of turtle trap construction as wellas a map of the Karkheh Regulating Dam Lake indicating
of side channels. Sixty percent of the study animals were
trapping locations.
caught within their subsequently defined 95% KDErange, 20% were caught in close proximity (5–20 m) to
using a randomized resampling approach with 10
their subsequently defined 95% KDE range, and only
iterations in the packages ‘‘adehabitat'' (Calenge 2006),
20% were caught at greater distances. Thirty percent of
‘‘maptools'' (Bivand and Lewin-Koh 2013), and ‘‘fields''
the study animals were caught within their core areas,
(Furrer et al. 2013) for Cran R (R Development Core
20% were caught in close proximity (5–20 m), and 50%
Team 2012) as described by Harris et al. (1990) and
were caught more than 20 m from their core areas.
Kernohan et al. (2001). In order to investigate whether
Home Range Size. — Due to transmitter failure in 4
turtles performed nomadic movements or exhibited site
cases, movement was analyzed for a total of 8 turtles.
fidelity, the radio-tracking data sets were compared with
Except for occasional basking, movement was exclusively
computer-simulated distribution models (Munger 1984;
aquatic (96%; n 5 254 total number of fixes).
Spencer et al. 1990; Turchin 1998; Schwarzkopf and
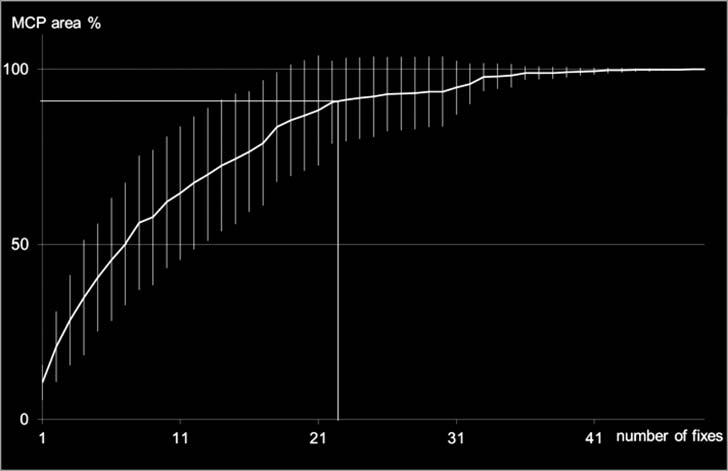
Incremental area analysis curves for turtles' MCPs
Alford 2002). ‘‘Random walk models'' (RWMs) were
revealed that 22 fixes were required to capture 90% of the
performed for each turtle using ‘‘turning angles'' and
study animals' home range size, suggesting the study
‘‘distances between successive fixes'' from real radio-
period was sufficient to obtain home range size estimates
tracking data using a bootstrapping approach with 100
(Fig. 6). Comparison of observed movements and results
iterations using the above packages for Cran R (Turchin
gained by the simulated RWMs revealed turtles' observed

GHAFFARI ET AL. — Home Range of Euphrates Softshell Turtle in Iran
Figure 5. Rafetus euphraticus with a very-high-frequency radio-tracking transmitter attached to its posterior carapace. Photograph byHanyeh Ghaffari.
movement patterns to be significantly smaller than
The number of fixes was highly variable among
random walk estimates, suggesting that turtles exhibited
individuals (range 20–51) and a linear regression analysis
site fidelity (Table 2).
between range size estimates and the number of fixes
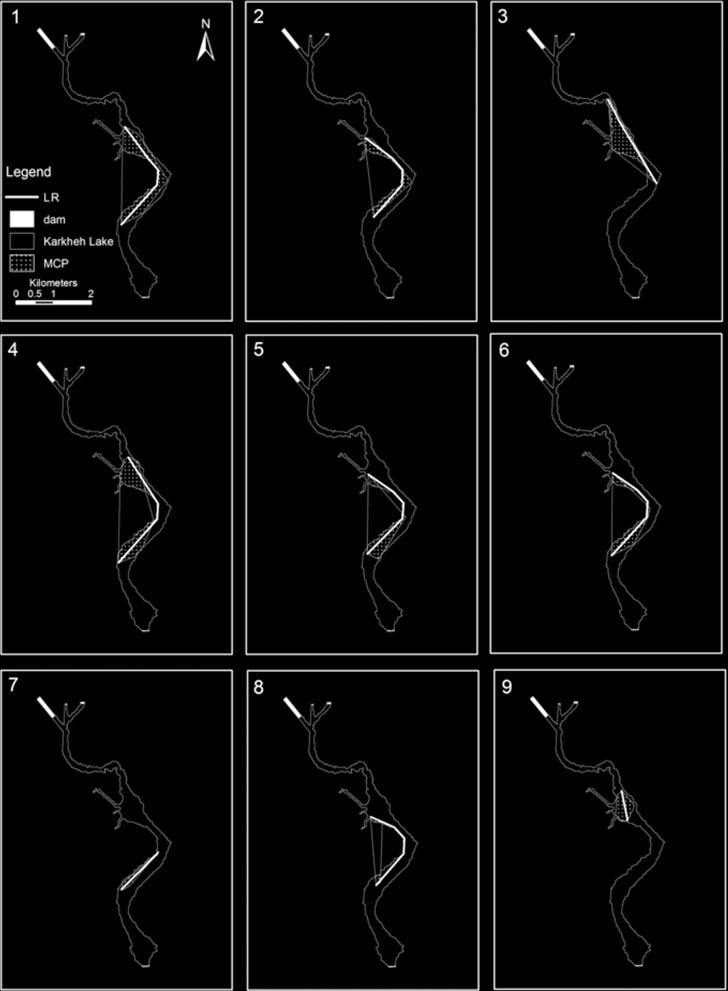
Mean LR size was 2.54 ± 0.83 km SD and ranged
obtained revealed a statistically significant bias (r2 5
from 0.80 to 3.41 km with a coefficient of variation (CV)
0.564, p 5 0.032, n 5 8). The turtles' mean total KDE
of 33% (Fig. 7). Mean RCA was 55.35 ± 17.98 ha SD
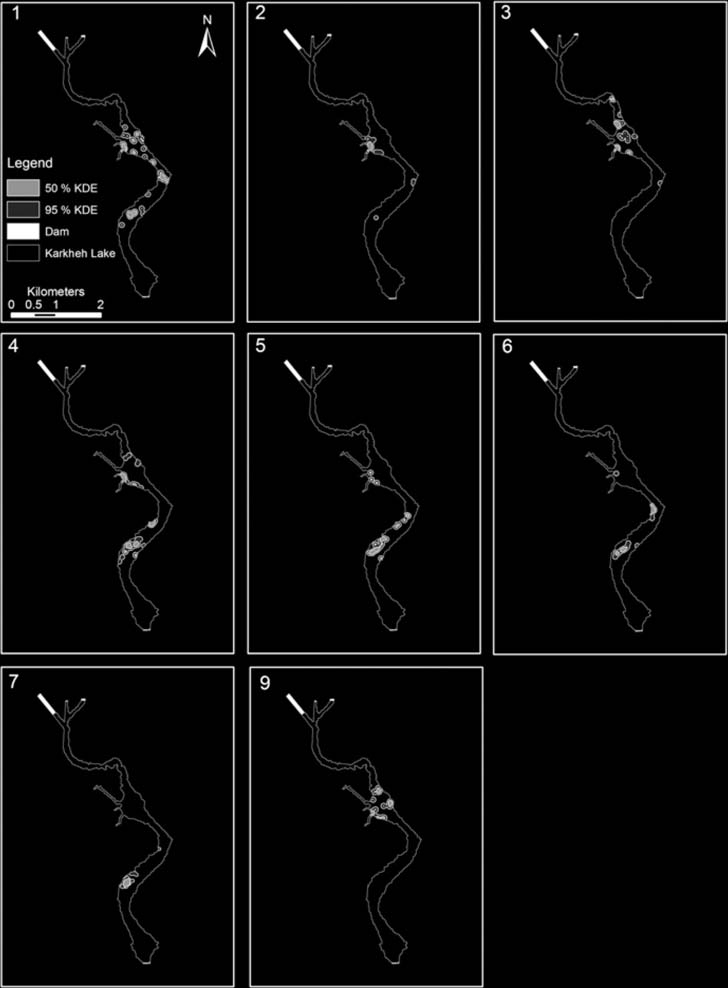
size was 21.75 ± 11.23 ha SD while individual 95%
and ranged from 17.38 to 71.24 ha (CV 5 32%). There
KDEs ranged from 9.04 to 39.51 ha (Fig. 8). While there
was no statistically significant relationship of LR/RCA
was no relationship between SCL and 95% KDE size
size with either SCL or BM (LR/RCA with SCL:
0.595, p 5 0.12, n 5 8), 95% KDE size was
0.517, p 5 0.15, n 5 9; LR/RCA with BM:
significantly related to BM (rs
0.886, p 5 0.019,
0.143, p 5 0.76, n 5 7). Sizes of MCPs varied
n 5 8). Mean core area was 5.74 ± 2.87 ha SD (range
2.59–9.91 ha; Fig. 8) and was significantly negatively
CV 5 49%) with a mean size of 47.49 ± 23.36 ha SD
related to both SCL and BM (50% KDE and SCL:
(Fig. 7). There was no significant relationship of MCP
0.714, p 5 0.047, n 5 8; 50% KDE and BM:
size with body size (MCP and SCL: r 5 2
0.943, p 5 0.005, n 5 6).
Home Range Overlap. — The MCP of each turtle
p 5 0.33, n 5 6).
overlapped with MCPs of 6–8 other turtles (mean 5
Table 1. Summary of turtles' body size and home range by minimum convex polygon (MCP), 95% and 50% kernel density estimator
(KDE) core areas, linear range (LR), and river channel area (RCA).
a SCL, straight-line carapace length.
CHELONIAN CONSERVATION AND BIOLOGY, Volume 13, Number 2 – 2014
Figure 6. The incremental area analysis plot illustrates that 22 fixes were needed to capture 90% of a study animal's minimum
convex polygon (MCP) home range size (horizontal bar). Vertical bars represent ranges of mean MCP area.
7.5 ± 0.71 SD, n 5 8; Table 3). The mean area of
and 4 (open, deep water) were below expected propor-
tions of use (Table 4). Analysis of habitat selection of
10.99 ± 3.92 ha SD (range 2.74–13.46 ha, n 5 6) and
individual turtles was not possible due to insufficient
32.47 ± 16.66 ha SD (range 5.08–61.05 ha, n 5 8).
numbers of observations (, 5 observations) in several
Total KDEs overlapped with 5–7 other turtles
habitat type categories.
(mean 5 6.31 ± 0.78 ha SD, n 5 8) with mean overlap
Basking and Nesting Habits. — Rafetus euphraticus
areas ranging from 4.75 ± 2.52 ha SD to 10.04 ± 4.25 ha
was observed basking along vegetated shorelines (35%),
SD among individuals (Table 3). Core areas overlapped
atop halms of Phragmites australis (30%), and on
with 4–7 core areas of other study animals (mean 5
floating trunks of fallen trees within dense foliage
5.50 ± 1.22 SD; n 5 8).
(20%). In addition, turtles were observed to bask
Habitat Selection. — Due to low sample sizes in
partially submerged on gravel along the shoreline
some habitats, habitat data from individual turtles were
(14%) and fully exposed on the muddy shoreline
pooled for analysis. Selection of habitat types was not
approximately 1 m from the water's edge (1%). During
proportional to availability (x2 5
2623, p , 0.0001).
basking, an individual's head and limbs were often
Bonferroni confidence intervals (95%) showed proportion
extended, as described by Gramentz (1991). One female
of use for habitat type 1 (vegetated shorelines) was greater
was observed nesting on the east shore by a local
than the expected proportion of use, whereas the
fisherman in 2011 but we could not find nests despite
proportions of use for habitat types 3 (floating vegetation)
Table 2. Range size estimates performed using kernel density estimators (KDEs, including the terrestrial portion) in comparison withrange sizes obtained from simulated random walk models. Results indicate Rafetus euphraticus possess home ranges, rather thanexhibiting a nomadic movement pattern.
95% KDE obs. (ha)
50% KDE obs. (ha)
a CI 95%595% confidence interval.
b Significant alteration of observed range size used and random walk model results. 2 5 site fidelity; 0 5 random movement; + 5 observed movementexceeds random walk predictions.
GHAFFARI ET AL. — Home Range of Euphrates Softshell Turtle in Iran
Figure 7. Map of the study area showing the turtles' linear range (LR) and minimum convex polygon (MCP) home range. Mapdesigned using ArcGis 9.3.
this study with those of previous studies. The MCPestimator is heavily influenced by outlying locations and
LR Size. — The only previously reported LR
therefore may incorporate areas that have never been used
estimates for trionychid turtles include the American
by the animal and as a consequence, often overestimates
species Apalone mutica (0.7 km in a small river; Plummer
range size (Powell 2000; Kenward 2001). According to
and Shirer 1975) and Apalone spinifera (1.5 km in a small
Borger et al. (2006), MCPs are subject to unpredictable
stream, Plummer et al. 1997; 11.1 km in a large river,
bias. Nilson et al. (2008) also questioned the ecological
Galois et al. 2002). Differences in sample sizes, study
value of the MCP. Nevertheless, the MCP is commonly
period, species, and habitat type hamper a direct
used to perform home range estimates and to facilitate
comparison among studies.
inter- and intraspecific comparison of different studies.
Home Range Sizes. — The use of different analytical
The KDE is currently the most widely used approach
methods complicates comparison of results obtained in
for home range estimates and habitat selection analysis
CHELONIAN CONSERVATION AND BIOLOGY, Volume 13, Number 2 – 2014
Figure 8. Map of the study area showing the turtles' 95% and 50% KDE home ranges. Individual no. 8 was excluded from the
analysis due to an insufficient number of fixes (n 5 6; minimum number of fixes required 5 20). Map designed using ArcGis 9.3.
(Worton 1995; Seaman and Powell 1996; Seaman et al.
significant relationships of home range sizes with BM
1999). However, the kernel technique may not accu-
and SCL, whereas these relationships could not be
rately estimate home range sizes for reptiles as the
demonstrated using the MCP method. Whereas range size
frequent multiple use of locations by an ectotherm leads
may depend on habitat quality and resource availability,
to autocorrelation (Row and Blouin-Demers 2006).
range shape and location may reflect resource distribution
Home range size is generally known to depend on the
and abundance (Bury 1979; Harestad and Bunnell 1979;
study animal's body size (Harestad and Bunnell 1979),
Savitz et al. 1983; Ims 1987; Macartney et al. 1988; Brown
which previous studies confirmed for several reptile
et al. 1994; Kenward 2001; Kjellander et al. 2004).
species, including aquatic chelonians (Schubauer et al.
Compared with studies conducted in relatively undisturbed
1990; Plummer et al. 1997; Perry and Garland 2002;
areas, Galois et al. (2002) suggested that range size might
Carrie re 2007). Despite the low sample size for R.
increase with increasing habitat fragmentation and modi-
euphraticus, the KDE method revealed statistically
fication, as in this study.
GHAFFARI ET AL. — Home Range of Euphrates Softshell Turtle in Iran
Plummer and Shirer (1975) and Galois et al. (2002)
Table 3. Overlap of movement areas of 8 Rafetus euphraticus,
reported females' ranges to be significantly larger than
given as percentage of the total range of the individuals listed inthe left column (see Table 1 for definition of abbreviations).
those of males or subadults in A. mutica and A. spinifera.
As we were unable to determine sex or reproductive
condition of turtles, their possible effects on range size in
R. euphraticus is unknown.
The data collection intervals in our study were highly
45.66 15.44 26.74
64.31 25.44 23.12
variable with several fixes obtained in a single day and
gaps of several weeks between subsequent field trips.
44.88 17.66 34.76
Since we required at least 20 fixes to calculate a home
range, we included all fixes in the analysis. Because this
0.00 100.00 100.00 100.00
inclusion likely resulted in an autocorrelated data set and
88.59 33.72 100.00
biased home range estimates (White and Garrott 1990),
results should be treated with caution.
Home Range Overlap. — As with R. euphraticus,
home ranges are known to overlap among individuals of
38.48 42.86 25.81 24.16
100.00 53.49 43.71 18.54
A. mutica and A. spinifera (Plummer and Shirer 1975;
Plummer et al. 1997). As a possible indicator of
50.77 26.83 20.08 25.51
intraspecific aggression in R. euphraticus, bite marks
44.33 30.66 30.66
along the posterior carapace edges have been reported by
0.00 70.70 85.54 65.70
Gramentz (1991) and along the lateral and caudal
33.21 100.00 43.96 17.61
carapace edges by Tas¸kavak and Atatu¨r (1995). Bite
marks were present in both sexes and different size andage classes (Gramentz 1991; Tas¸kavak and Atatu¨r 1995).
Bite marks commonly occur on the posterior edge of the
40.45 14.88 17.70
carapace of male A. mutica and A. spinifera and are
related to courtship aggression by females (Plummer
1977b; M.V. Plummer, pers. obs.). We found few bite
marks along the lateral carapace edge of adult R.
26.10 58.40 35.24
euphraticus in our study, suggesting either lower levels
of aggression or lower population density. Trappingsuccess in the present study was low in comparison withstudies of R. euphraticus in Turkey (Tas¸kavak et al., inpress), also suggesting either low population density or a
The only areal ranges reported for a trionychid turtle
reluctance to enter traps.
are those for A. spinifera in a small stream (11.6 ha;
Habitat Selection. — In Turkey and Iran, R.
Plummer et al. 1997) and a large river (2424 ha; Galois
euphraticus generally inhabits calm and shallow rivers,
et al. 2002). The mean river channel area (55.35 ha) as
preferring tributaries and the shallow backwaters of main
well as the slightly smaller mean MCP 100% home range
river channels and seasonal ponds and wetlands. Habitat
(47.49 ha) for R. euphraticus in a much wider lake is
preferences may differ between adults and juveniles
comparable to the 95% MCP reported by Galois et al.
(Gramentz 1991; Tas¸kavak and Atatu¨r 1995, 1998;
(2002). Correlation of habitat size and range size in
Ghaffari et al. 2008; Tas¸kavak et al., in press). Adults
freshwater turtles has previously been reported (Plummer
preferred tributaries with access to deeper water (up to
et al. 1997).
2 m), whereas juveniles preferred puddles (10–15 cm
Table 4. Habitat selection of Rafetus euphraticus.
True proportion of
observations (pi)
a 95% confidence interval of area under an expected selection hypothesis.
b Significant preference for habitat type: + 5 significantly higher than expected; 2 5 significantly below expected; ns 5 not significant.
CHELONIAN CONSERVATION AND BIOLOGY, Volume 13, Number 2 – 2014
deep) with higher water temperatures and abundant
strongly affect freshwater turtle populations (Dodd
potential prey (Gramentz 1991; Tas¸kavak and Atatu¨r
1990; Gramentz 1993; Tas¸kavak and Atatu¨r 1995,
1995). Our results show that R. euphraticus favored
1998). Severe population decline as a response to dam
vegetated shorelines over open deep water in concor-
constructions on the Euphrates River was reported by
dance with the previous studies in Turkey. While
Gramentz (1993) and Tas¸kavak and Atatu¨r (1998).
vegetated shorelines are essential for nesting (Ghaffari
Channelization and dam construction were also found to
et al. 2013), vegetated edges may serve as refuge in
heavily fragment remnant populations of R. euphraticus
disturbed habitats such as reservoir lakes. Therefore the
(Ihlow et al. 2014). Currently R. euphraticus is threatened
presence of such vegetated shorelines is considered
by the construction of several additional dams across its
an important feature, providing retreats for the endan-
range, which will cause further habitat fragmentation and
gered species, especially in disturbed habitats. The
loss and may even increase the probability of local
preference for shoreline habitat may be related to the
extinction (Gramentz 1991). In addition, the species is
higher water temperatures at the edges or activity levels.
affected by water pollution through pesticides, fertilizers,
For example, foraging individuals might select areas of
oil, garbage, and industrial chemicals (Ghaffari et al. 2008).
higher food abundance (plant material, insect larvae,
Turtles are frequently caught accidentally on baited hooks
crustaceans, mollusks, amphibians, and fish) within the
or entangle themselves in fishing nets (Ghaffari et al.
dense Phragmites australis stands along the lakes edges
2008). Despite fishing being prohibited in April and May in
compared to deep open water, whereas inactive individ-
Khuzestan Province, people were observed fishing
uals might select areas based on suitability of retreat sites
throughout the year, sometimes even using illegal electro-
(Siebenrock 1913; Tas¸kavak and Atatu¨r 1998). We had
fishing (H. Ghaffari, pers. obs.). Because turtles are
difficulties determining the activity level of animals in
wrongly believed to be detrimental to fish populations,
dense vegetation as they were easily disturbed when
they are often killed by fishermen (Ghaffari et al. 2008).
approached. Additional research needs to be done to
As the endangered species' survival may soon
clarify this issue.
become critical, knowledge of its ecology is desperately
Although the results of statistical analysis generally
needed to prepare a conservation management plan for the
agreed with observations of habitat selection made,
species. To successfully sustain viable populations,
avoidance of habitat type 3 (floating vegetation) does
hunting, fishing, and pollution need to be reduced to a
not. The distribution and abundance of vegetation at the
minimum while patrolling needs to be initiated. Consid-
KRDL is known to be highly variable among seasons and
ering our results on range sizes and habitat selection,
years. The satellite images used were taken in 2007 and
future conservation efforts should focus on large but
vegetation cover likely has changed since dam construc-
shallow interconnected wetlands and rivers with side
tion. In addition, habitat selection analysis procedure
channels and backwaters. Regarding the increasing
requires that temporal spacing between observations is
modification of natural rivers, artificial habitats consid-
free from autocorrelation (Byers and Steinhorst 1984),
ered suitable for R. euphraticus should provide unvege-
which unfortunately was not the case in this study.
tated water edges as well as retreat sites covered with
Gramentz (1991) suggested habitat use and selection
vegetation. To prevent further fragmentation of popula-
might vary seasonally in R. euphraticus. Unfortunately,
tions through dams, future dam constructions should be
data spanning all seasons in this study were few,
equipped with passes for turtles and other aquatic species
especially for the winter. Likewise, although sexual
to facilitate emigration (Ihlow et al. 2014).
differences in habitat selection in softshell turtles are
To establish successful conservation management,
known (Plummer 1977a), no comparable data for R.
we consider capacity-building and education of the native
euphraticus were collected.
populations to be highly important. A program to protect
Basking Habits. — Basking of individuals or groups
the Euphrates softshell turtle populations in Khuzestan
of up to 10 R. euphraticus was observed by Griehl (1981).
Province was carried out by the Pars Herpetologists
In concordance with Gramentz (1991), basking was
Institute from 2009 to 2012 through a partnership program
frequently observed close to the water's edge, mostly on
with the Global Environment Facility funded by the Small
the muddy shore but also on grass or stone. Turtles in this
Grants Programme of the United Nations Development
study tended to bask in more hidden places such as
Programme. The project focused on education and raising
vegetated shorelines, floating tree trunks, and floating
awareness and highlighting the necessity of conservation
vegetation, which may be related to frequent disturbance
measures to protect and conserve the Euphrates softshell
by fishermen.
turtle. This project already has proven successful and
Conservation Status. — Recent regulations of rivers
induced a significant behavioral change among the local
for flood control and hydroelectric power have severely
population, providing confidence for future projects. The
altered environmental conditions (Partow 2001). Water
establishment of the introduced softshell turtle species
level fluctuation and decreasing temperatures have been
Pelodiscus sinensis, abandoned from the pet trade, may
reported to cause the depletion of food items and
become another threat for the species in the near future.
induce changes in aquatic and riverine vegetation that
Although R. euphraticus is not consumed by native
GHAFFARI ET AL. — Home Range of Euphrates Softshell Turtle in Iran
Iranians, Chinese employees of the National Iranian Oil
BORGER, L., FRANCONI, N., DE MICHELLE, G., GANTZ, A., MESCHI,
Company catch the species for human consumption,
F., MANICA, A., LOVARI, S., AND COULSON, T. 2006. Effects of
especially in the Hawr-al-Azim wetland and along the
sampling regime on the mean variance of home range sizeestimates. Journal of Animal Ecology 75:1493–1405.
border with Iraq.
BROWN, G.P., BISHOP, C.A., AND BROOKS, R.J. 1994. Growth rate,
reproductive output, and temperature selection of snapping
turtles in habitats of different productivities. Journal ofHerpetology 28:405–410.
We appreciate the kind help of Farhad Gholinejad
BURY, R.B. 1979. Population ecology of freshwater turtles. In:
(head of the Department of Environment of Dezful
Harless, M. and Morlock, H. (Eds.). Turtles: Perspectives and
County). We are especially grateful to Hormoz Mah-
Research. New York: John Wiley and Sons, pp. 571–410.
moudi-Rad (head of the Department of Environment of
BYERS, C.R. AND STEINHORST, R.K. 1984. Clarification of a
Khuzestan Province) for kindly issuing the relevant
technique for analysis of utilization-availability data. Journal
permits. We are indebted to Behrooz Nejati (former head
of Wildlife Management 48:1050–1053.
of Dez National Park), Mehrshad Ahmadvand (current
CALENGE, C. 2006. The package adehabitat for the R software: a
head of Dez National Park), and Ali Fathinia (head of the
tool for the analysis of space and habitat use by animals.
Ecological Modelling 197:516–519.
Department of Environment of Andimeshk City) for their
CARRIE RE, M.A. 2007. Movement patterns and habitat selection
generous support. We are indebted to Mirza Ali Shanbool
of common map turtles (Graptemys geographica) in St.
for providing valuable information, assistance, and his
Lawrence Islands National Park, Ontario, Canada. MSc
boat during the fieldwork. Furthermore we thank Faraham
Thesis, University of Ottawa, Ottawa, Canada.
Ahmadzadeh for his kind assistance. We are indebted to
DODD, K. 1990. Effects of habitat fragmentation on a stream-
Mansour and Mehdi Shalageh, Seyed Morteza Tafakh,
dwelling species, the flattened musk turtle Sternotherus
and Ahmad Zobeidi Rad (Dez and Karkheh Environment
depressus. Biological Conservation 54:33–45.
Protection Guards) for their help during the field surveys.
DOODY, J.S., YOUNG, J.E., AND GEORGES, A. 2002. Sex differences
in activity and movements in the pig-nosed turtle, Caretto-
We are especially grateful to Dr Amir Rostami, Dr
chelys insculpta, in the wet–dry tropics of Australia. Copeia
Alireza Vajhi, Dr Mohsen Paknejad, and Dr Iman
Memarian for their kind help during the transmitter
DRESLIK, M.J., KUHNS, A.R., PHILLIPS, C.A., AND JELLEN, B.C.
attachment procedure. In addition, we want to express our
2003. Summer movements and home range of the cooter
gratitude to Majid and Amin Shanbool, Alireza Shahrdari
turtle, Pseudemys concinna, in Illinois. Chelonian Conserva-
Panah, Ahmad Havani, Hadi Fahimi, Parham Dibadj, and
tion and Biology 4:706–710.
Farhad Ghaffari for their kind support during the
FURRER, R., NYCHKA, D., AND SAIN, S. 2013. Fields: tools for
fieldwork. We thank Cle´ment Calenge for valuable
spatial data. R package version 6.7.6. http://CRAN.R-project.
org/package5fields (12 March 2013).
comments on our R script and Ursula Bott for proofread-
GALOIS, P., LE´VEILLE´, M., BOUTHILLIER, L., DAIGLE, C., AND
ing the draft of this manuscript. Finally we wish to
PARREN, S. 2002. Movement patterns, activity and home
acknowledge several other people who assisted in various
range of the eastern spiny softshell turtle (Apalone spinifera)
ways: Laleh Daraie (Global Environment Facility/Small
in northern Lake Champlain, Que´bec, Vermont. Journal of
Grants Programme National Coordinator) for her support,
Shahab Cheraghi for his expert advice, Valiolah Mozaf-
GEFFEN, E. AND MENDELSSOHN, H. 1988. Home range use and
farian for identifying plants, and Nooshin Satei, Arzhic
seasonal movements of the Egyptian tortoise (Testudo
Basirov, and Nazli Khajehnejadian. Funding for the
kleinmanni) in the northwestern Negev, Israel. Herpetologica44:354–359.
radiotelemetry equipment was kindly provided by the
GHAFFARI, H., PLUMMER, M.V., KARAMI, M., MAHROO, B.S.,
Rufford Small Grants for Nature Conservation (no. 7916-
AHMADZADEH, F. AND RO¨DDER, D. 2013. Notes on a nest and
1), for which we are very grateful.
emergence of hatchlings of the Euphrates softshell turtle(Rafetus euphraticus) at the Dez River, Iran. Chelonian
Conservation and Biology 12:319–323.
GHAFFARI, H., TAS¸KAVAK, E., AND KARAMI, M. 2008. Conservation
ANONYMOUS. 1987. Guidelines for use of live amphibians and
status of the Euphrates softshell turtle, Rafetus euphraticus, in
reptiles in field research. Society of Ichthyologists and
Iran. Chelonian Conservation and Biology 7:223–229.
Herpetologists, The Herpetologists' League, Society for the
GRAMENTZ, D. 1991. Beobachtungen an der Euphrat-Weichschildkro
Study of Amphibians and Reptiles. www.asih.org/sites/
Trionyx euphraticus (Daudin, 1802) in Ost-Anatolien. Salamandra
pdf (15 September 2011).
GRAMENTZ, D. 1993. Vernichtung einer Population von Rafetus
BEYER, H.L. 2004. Hawth's analysis tools for ArcGIS. www.
euphraticus am Oberlauf des Euphrat. Salamandra 29:86–89.
spatialecology.com/htools (20 October 2012).
GRIEHL, K. 1981. Reptilien in Anatolien. Sielmanns Tierwelt 5:
BIRICIK, M. AND TURG˘A, S. 2011. Description of an Euphrates
softshell turtle (Rafetus euphraticus) nest from the Tigris
HARESTAD, A.S. AND BUNNELL, F.L. 1979. Home range and body
River (SE Turkey). Salamandra 47:99–102.
weight—a reevaluation. Ecology 60:389–402.
BIVAND, R. AND LEWIN-KOH, N. 2013. Maptools: tools for reading
HARRIS, S., CRESSWELL, W.J., FORDE, P.G., TREWHELLA, W.J.,
and handling spatial objects. R package version 0.8-23. http://
WOOLLARD, T., AND WRAY, S. 1990. Home-range analysis
CRAN.R-project.org/package5maptools (20 March 2013).
using radio-tracking data—a review of problems and
CHELONIAN CONSERVATION AND BIOLOGY, Volume 13, Number 2 – 2014
techniques particularly as applied to the study of mammals.
PERRY, G. AND GARLAND, T., JR. 2002. Lizard home ranges
Mammal Review 20:91–123.
revisited: effects of sex, body size, diet, habitat, and
HORNE, J.S. AND GARTON, E.O. 2009. Animal space use 1.3.
phylogeny. Ecology 83:1870–1885.
www.cnr.uidaho.edu (20 October 2012).
PITTMAN, S.E. AND DORCAS, M.E. 2009. Movement, habitat use,
IHLOW, F., AHMADZADEH, F., GHAFFARI, H., TAS¸KAVAK, E.,
and thermal ecology of an isolated population of bog turtles
HARTMANN, T., ETZBAUER, C. AND RO¨DDER, D. 2014. Assess-
(Glyptemys muhlenbergii). Copeia 2009:781–790.
ment of genetic variation, habitat suitability and effectiveness
PLUMMER, M.V. 1977a. Activity, habitat and population structure
of reserves for future conservation planning of the Euphrates
in the turtle, Trionyx muticus. Copeia 1977:431–440.
soft-shelled turtle Rafetus euphraticus (Daudin, 1802).
PLUMMER, M.V. 1977b. Notes on the courtship and mating
Aquatic Conservation: Marine and Freshwater Ecosystems.
behavior of the softshell turtle, Trionyx muticus (Reptilia,
Testudines, Trionychidae). Journal of Herpetology 1:90–92.
IMS, R.A. 1987. Male spacing systems in microtine rodents.
PLUMMER, M.V. 2008. A notching system for marking softshell
American Naturalist 130:475–484.
turtles. Herpetological Review 39:64–65.
NTERNATIONAL UNION FOR CONSERVATION OF NATURE (IUCN).
LUMMER, M.V., MILLS, N.E., AND ALLEN, S.L. 1997. Activity,
2013. Red List of Threatened Species. Version 2012.2. www.
habitat, and movement patterns of softshell turtles (Trionyx
iucnredlist.org (20 May 2013).
spiniferus) in a small stream. Chelonian Conservation and
ARVIS, A., REUTER, H.I., NELSON, A., AND GUEVARA, E. 2008.
Hole-filled seamless SRTM data V4. International Centre for
PLUMMER, M.V. AND SHIRER, H.W. 1975. Movement patterns in a
Tropical Agriculture (CIAT). http://srtm.csi.cgiar.org (15
river population of the softshell turtle, Trionyx muticus.
University of Kansas Museum of Natural History Occasional
Papers 43:1–26.
ENRICH, R.I. AND TURNER, F.B. 1969. Measurement of non-
circular home range. Journal of Theoretical Biology 22:227–
PLUTO, T.G. AND BELLIS, E.D. 1988. Seasonal and annual
movements of riverine map turtles, Graptemys geographica.
Journal of Herpetology 22:152–158.
AY, W.R. 2004. Movements and home ranges of radio-tracked
Crocodylus porosus in the Cambridge Gulf region of Western
POWELL, R.A. 2000. Animal home ranges and territories and
Australia. Wildlife Research 31:495–508.
home range estimators. In: Boitani, L. and Fuller, T. (Eds.).
Research Techniques in Animal Ecology: Controversies
KENWARD, R.E. 2001. A Manual for Wildlife Radio Tagging.
and Consequences. New York: Columbia University Press,
London: Academic Press, 313 pp.
pp. 65–110.
KERNOHAN, B.J., GITZEN, R.A., AND MILLSPAUGH, J.J. 2001.
R DEVELOPMENT CORE TEAM. 2012. R: a language and
Analysis of animal space use and movement. In: Millspaugh,
environment for statistical computing. www.R-project.org
J.J. and Marzluff, J.M. (Eds.). Radio Tracking and Animal
(15 February 2013).
Population. San Diego: Academic Press, pp. 125–166.
ROW, J.R. AND BLOUIN-DEMERS, G. 2006. Kernels are not accurate
KJELLANDER, P., HEWISON, A.J.M., LIBERG, O., ANGIBAULT, J.M.,
estimators of home-range size for herpetofauna. Copeia 2006:
BIDEAU, E., AND CARGNELUTTI, B. 2004. Experimental evidence
for density-dependence of home-range size in roe deer
RYAN, S.J., KNECHTEL, U.C., AND GETZ, W.M. 2006. Range and
(Capreolus capreolus): a comparison of two long-term
habitat selection of African buffalo in South Africa. Journal
studies. Oecologia 139:78–485.
of Wildlife Management 70:764–776.
KUCHLING, G. 2003. A new underwater trap for catching turtles.
SAVITZ, J., FISH, P.A., AND WESZELY, R. 1983. Effects of forage
Herpetological Review 34:126–128.
on home-range size of largemouth bass. Transactions of the
LUE, K.Y. AND CHEN, T.H. 1999. Activity, movement patterns,
American Fisheries Society 112:772–776.
and home range of the yellow-margined box turtle (Cuora
SCHUBAUER, J.P., GIBBONS, J.W., AND SPOTILA, J.R. 1990. Home
flavomarginata) in northern Taiwan. Journal of Herpetology
range and movement patterns of slider turtles inhabiting Par
Pond. In: Gibbons, J.W. (Ed.). Life History and Ecology of
MACaRTNEY, J.M., GREGORY, P.T., AND LARSEN, K.W. 1988. A
the Slider Turtle. Washington, DC: Smithsonian Institution
tabular survey of data on movements and home ranges of
Press, pp. 223–232.
snakes. Journal of Herpetology 22:61–73.
SCHWARZKOPF, L. AND ALFORD, R.A. 2002. Nomadic movement in
MANLY, B.F.J., MCDONALD, L.L., THOMAS, D.L., MCDONALD,
tropical toads. Oikos 96:492–506.
T.L., AND ERICKSON, W.P. 2002. Resource Selection by
SEAMAN, D.E., MILLSPAUGH, J.J., KERNOHAN, B.J., BRUNDIGE, G.C.,
Animals: Statistical Design and Analysis for Field Studies.
RAEDEKE, K.J., AND GITZEN, R.E. 1999. Effects of sample size
Dordrecht, Netherlands: Springer, 221 pp.
on kernel home range estimates. Journal of Wildlife
MOHR, C.O. 1947. Table of equivalent populations of North
American small mammals. The American Midland Naturalist
SEAMAN, D.E. AND POWELL, R.A. 1996. An evaluation of the
accuracy of kernel density estimators for home range
MUNGER, J.C. 1984. Home ranges of horned lizards (Phryno-
analysis. Ecology 77:2075–2085.
soma): circumscribed and exclusive? Oecologica 62:351–360.
SEXTON, O.J. 1959. Spatial and temporal movements of a
NEU, C.W., BYERS, R., AND PEEK, J.M. 1974. A technique for
population of the painted turtle, Chrysemys picta marginata
analysis of utilization–availability data. Journal of Wildlife
(Agassiz). Ecological Monographs 29:113–140.
SIEBENROCK, F. 1913. Schildkro¨ten aus Syrien und Mesopota-
NILSON, E.B., PEDERSEN, S., AND LINNELL, J.D.C. 2008. Can
mien. Annalen des Naturhistorischen Museums in Wien 27:
minimum convex polygon home ranges be used to draw
biological meaningful conclusions? Ecological Research 23:
SOUZA, F.L., RAIZER, J., DA COSTA, H.T.M., AND MARTINS, F.I.
2008. Dispersal of Phrynops geoffroanus (Chelidae) in an
PARTOW, H. 2001. The Mesopotamian marshlands: demise of an
urban river in central Brazil. Chelonian Conservation and
ecosystem. www.grid.unep.ch (20 May 2013).
GHAFFARI ET AL. — Home Range of Euphrates Softshell Turtle in Iran
SPENCER, S.R., CAMERON, G.N., AND SWIHART, R.W. 1990.
P.C.H. and Rhodin, A.G.J. (Eds.). The Conservation Biology of
Operationally defining home range: temporal dependence
Freshwater Turtles. Lunenburg, MA: Chelonian Research
exhibited by hispid cotton rats. Ecology 71:1817–1822.
Foundation. In press.
TAS¸KAVAK, E. AND ATATU¨R, M.K. 1995. Threats to survival of
TURCHIN, P. 1998. Quantitative Analysis of Movement: Measur-
Euphrates soft-shelled turtle (Rafetus euphraticus; Daudin,
ing and Modeling Population Redistribution in Animals and
1802) in Southeastern Anatolia. In: Smith, S.S. and Smith,
Plants. Sunderland, MA: Sinauer Associates, 369 pp.
S.S. (Eds.). Proceedings of the International Congress of
WHITE, G.C. AND GARROTT, R.A. 1990. Analysis of Wildlife
Chelonian Conservation, pp.141–145.
Radio-Tracking Data. San Diego: Academic Press, 384 pp.
TAS¸KAVAK, E. AND ATATU¨R, M.K. 1998. Distribution and habitats
WORTON, B.J. 1995. Using Monte-Carlo simulation to evaluate
of the Euphrates softshell turtle, Rafetus euphraticus (Daudin,
kernel-based home-range estimators. Journal of Wildlife
1802) in Southeastern Anatolia; with some observations on
biology and factors endangering its survival. ChelonianConservation and Biology 3:20–30.
Received: 20 July 2013
TAS¸KAVAK, E., ATATU¨R, M.K., AND MEYLAN, P. Rafetus euphraticus
Revised and Accepted: 30 September 2013
(Daudin, 1802) – Euphrates Soft-Shelled Turtle. In: Pritchard,
Handling Editors: Peter V. Lindeman
Source: http://www.pars-herp.ir/images/Papers/ScientificPapers/Ghaffarietal2014.pdf
zdravotnipojistenci.cz
Tender systems for outpatient pharmaceuticals in the European Union: Evidence from the Netherlands and Germany Panos Kanavos with the assistance of Alessandra Ferrario Elena Nicod and Dale Sandberg LSE Health London School of Economics FOR THE EUROPEAN COMMISSION - DG ENTERPRISE
Chapter 14 veterinary aspects
COMMISSIONED PAPER (UK) This paper was commissioned by FECAVA for the Special issue of EJCAP, Genetic/Hereditary Disease and Breeding. Must not be copied without permission © 2014 Chiari–like malformation and syringomyelia Clare Rusbridge Introduction Syringomyelia is a condition characterised by fluid filed cavities (syrinxes or syringes) within the central spinal