Dspace.library.daffodilvarsity.edu.bd

Performance Evaluation of Different Brands
of Pantoprazole Tablets
Submitted by
Md. Rasel Mamun
ID NO: 101-29-157
Department of Pharmacy
Daffodil International University
Supervised by
Sharifa Sultana
Department of Pharmacy
Daffodil International University
Pharmacy Department
Faculty of Allied Health Science
Daffodil International University
This is to certify that the results of the investigation that are embodied in this project are
original and have not been submitted before in substance for any degree or diploma of
this university. The entire present work submitted as a project work for the partial
fulfillment of the degree of bachelor of pharmacy, is based on the result of author's (Md.
Rasel Mamun, ID NO: 101-29-157) own investigation.
Sharifa Sultana Lecturer Department of Pharmacy Faculty of Allied Health Science Daffodil International University
I would like to express my deep praise to the Almighty Allah who has given me the
ability to complete my project work and the opportunity to study in this subject.
I am very much grateful to my honorable project supervisor Sharifa Sultana , Lecturer,
Daffodil International University for her excellent guidance and constant supervision as
well as for providing necessary information regarding the project & also for her support
in completing the project
I would like to express my humble regards to Md. Arifur Rahman Fahim, Assistant
Professor & Head, Department of Pharmacy, Daffodil International University.
I also wish to offer my respect to all of the teachers of Pharmacy Department, Daffodil
International and thankful to other members for their excellent co-operation with us.
I am highly indebted to the Authority of Daffodil International University for their
guidance and constant supervision as well as for providing necessary information
regarding the project & also for their support in completing the project.
I am also grateful to my group members (Sumaiya Parvin, Jhumpa Raha, Nafisa
Yesmin) for their nice co-operation with me in completing my project.
I am also greatful to my friends especially Md. Riadh Hasan Rana, Md. Fahmid
Hasan, Mohammad Fariz Uddin, S.M Farid Imam, Sujan Sarker, Sakibur Rahman
for giving me mental support to complete my project work and paper.
Finally, I would like to express my gratitude towards my Parents & Other family
members for their kind co-operation and encouragement which help me in completion of
Dedicated to……
My Family Members
Abstract
Pantoprazole is a widely produced and marketed drug by many Pharmaceutical
companies in Bangladesh. This study is done to compare the evaluation parameter
(friability, weight variation, assay, disintegration and dissolution) of enteric coated
Pantoprazole tablets. Different brands of Pantoprazole tablets of top, middle and lower
listed company were collected from retail pharmacy of Bangladesh Market for their
evaluation test. Specified method of USP is followed for their evaluation test. RSD value
of weight variation of different drugs is in the range of (0.78-1.65)%, maximum friability
of the tablets of all brands is 0.4%, maximum disintegration time in phosphate buffer is
17.42 minutes, Maximum average potency among the brands is 98.14 % and minimum
potency is 94.60%, among the brands the maximum drug release in 0.1N HCl is 6.06 ,
minimum dissolution in phosphate buffer after 45 minutes is 79.94% and the maximum is
88.03%. The result of friability, weight variation, and assay and disintegration tests of all
marketed products comply with pharmacopoeial limit except some parameter in case of
lower listed company. This study is done to view the scenario of the quality of different
brands of Pantoprazole tablets in Bangladesh market.
Keywords: Enteric coated tablet, Pantoprazole, Proton pump inhibitors, Weight
variation, Disintegration, Dissolution.
Chapter one – Introduction
Definition of tablet
Classification of tablet
Properties of tablet
Advantage of tablet
Quality & criteria of drugs
Evaluation Parameter of tablets
Weight variation test
Disintegration test
Assay / Potency test
Factors / source of quality variation
History of Pantoprazole
Pharmacology of Pantoprazole
Some market preparation available in Bangladesh
Chapter Two- Materials & Methods
Materials
Collection of samples
Collection of standard
Coding of tablets
Labeling on the inner cartoon of the collected samples
Reagents used for the study
Instruments used for the study
Physical analysis
Weight variation test
Friability test of tablets
Disintegration test of tablets
Dissolution rate test of tablets
Chemical analysis
Preparation of standard curve of pantoprazole
Assay / Potency test
Chapter Three - Results and discussion
Result & discussion of weight variation test
Result & discussion of friability test
Result & discussion of disintegration test
Result & discussion of assay / potency test
Result & discussion of dissolution test
List of tables
Adverse drug reaction reported in clinical trial of adult patients 15
with GERD at a frequency of > 2%
Weight variation of P01
Weight variation of P02
Weight variation of P03
Weight variation of P04
Weight variation of P05
Friability of various brands of Pantoprazole
Disintegration of various brands of Pantoprazole
Dissolution rate of different brands of Pantoprazole tablets in
Dissolution rate of P01
Dissolution rate of P02
Dissolution rate of P03
Dissolution rate of P04
Dissolution rate of P05
List of figures
Figure No.
Structure of Pantoprazole
3 – D structure of Pantoprazole
Mechanism of acid inhibition
Analytical balance used for weight variation test
Friability test apparatus for friability test
Disintegration apparatus used for disintegration test
Dissolution apparatus used for dissolution test
Standard curve of Pantoprazole
UV spectroscopy used to measure absorbance
Friability of various brands Pantoprazole tablets
Disintegration time of various brands of Pantoprazole 47
Average potency of different market preparations of 50
Pantoprazole tablets
Dissolution rate of various brands of Pantoprazole 50
tablets in 0.1N HCl after 2 hrs.
Dissolution rate of various brands of Pantoprazole 52
tablets in phosphate buffer
Chapter One
"Daffodil International University"
1.1 An Overview
Bangladesh with a population of about 166 million is one of the developing countries of
South Asia and is actively involved in the Action Program of Essential Drugs proposed by
WHO. Through a developing country, over the last few years, Bangladesh has shown
commendable development in the pharmaceutical sector. About 300 pharmaceutical
companies are operating at the moment. Current market size is approximately 30,000 million
taka per year. Only 3% of the drugs are imported, the remaining 97% come from local
companies. Positive developments in the pharmaceutical sector have enabled Bangladesh to
export medicine to global markets. At present, Bangladesh's pharmaceutical industry is
effectively exporting their products to 79 countries. The number is expected to grow in the
coming months. In addition to regular products like tablets or capsules; HFA inhalers, nasal
sprays, IV infusions and other high-tech products are being exported from the country.
Bangladesh's pharmaceutical products in every way, meet international standard.
Actual growth of pharmaceutical industry in the country started in 1982, when the Drug
Control Ordinance was promulgated. The restriction of disproportionate import of drugs
encouraged local companies to increase production of their own products. Although this
displeased the multinational companies those were importing medicines to Bangladesh, the
regulation accelerated growth of local companies.
Pantoprazole is in a group of drugs called proton pump inhibitors. It decreases the amount of
acid produced in the stomach. Pantoprazole is used to treat erosive esophagitis (damage to the
esophagus from stomach acid), and other conditions involving excess stomach acid such as
Zollinger-Ellison syndrome. Pantoprazole is not for immediate relief of heartburn symptoms.
Pantoprazole may also be used for purposes not listed in this medication guide. Pantoprazole
is not for immediate relief of heartburn symptoms.
"Daffodil International University"
1.2 Tablet
Tablets are solid preparations each containing a single dose of one or more active ingredients
and obtained by compressing uniform volumes of particles. They are intended for oral
administration. Some are swallowed whole, some are after being chewed, some are dissolved
or dispersed in water before being administered and some are retained in the mouth where the
active ingredients liberated.
Tablets are usually circular solid cylinders, the end surfaces of which are flat or convex.
These are the most widely used solid dosage form medicaments because they offer a number
of advantages to the patient, prescriber, manufacturer to the patient, prescriber, manufacture
and the manufacturing pharmacist. Because of these advantages their popularity is
continuously increasing day by day.
1.2.1 Classification of Tablets
Mainly tablets are classified into two classes
A. Compressed tablets
B. Molded tablets
1.2.1.1 Compressed tablets
The compressed tablets usually prepared on large scale production methods, whereas the
molded tablets are prepared extemporaneously on small scale . This two main type of
tablet are further classified as follows :
1. Chewable tablets
2. Sublingual tablets
3. Effervescent tablets
4. Soluble tablets
5. Dispersible tablets
6. Gastro-resistant tablets
7. Modified release tablets
8. Tablets for use in the mouth
10. Soluble tablets
11. Layered tablets
"Daffodil International University"
1.2.1.2 Molded tablets
1. Hypodermic tablets
2. Dispensing tablets
1.2.2 Properties of a Good Tablet
Ø It should be accurate and uniform in weight.
Ø The size and shape should be reasonable for easy administration.
Ø The tablets should not be too hard to disintegrate in the stomach.
Ø There should not be any incompatibilities.
Ø They should be chemically and physically stable during storage.
Ø They should not break during transportation or crumble in the hands of the patient.
Ø They should be attractive in appearance.
Ø There should not be any manufacturing defects like cracking or chipping or
Ø They should be easy and economical in production.
Ø After administration, it should disintegrate readily.
1.2.3 Advantages
Ø Tablets have the following advantages
Ø They are easy to swallow
Ø They are easy to carry
Ø They are attractive in appearance
Ø Sugar coating can mask unpleasant taste
Ø They don't require any measurement of dose. The strip or blister packing has further
facilitated the process of taking the dose by the patient. Moreover it providing a sealed
covering which protects the tablets from atmospheric conditions likes as air, moisture and
Ø Some of the tablets are provided into halves and quarters by drawing lines during
manufacturing to facilitate breakage whenever a fractional dose is required.
Ø An accurate amount of medicament even if very small can be incorporated
Ø Tablets provide prolonged stability to medicament
Ø The incompatibilities of medicaments and their deterioration due to environmental
factors are less in tablet form.
"Daffodil International University"
Ø Since they are produced on a large scale therefore their cost of production is relatively
low, hence economical.
1.3 Quality and Its Criteria
Quality is an absolute necessity for medicines. The quality of drugs means quality of
treatment that ensures the well being of the patients. According to the WHO (World Health
Organization), the manufacturers must assume responsibility for the quality of the drugs he
produces. A medicinal product must satisfy certain pharmacopoeial standards to claim it to be
a quality drug. The principal criteria for a quality drug product are shown in figure-1.1
Regulatory
Aim of Quality
Compliance
Stability
Efficacy
Fig.1.1: The aim for Quality.
Ø Safety
Safety of medicine implies that the drug substance must meet certain safety requirements
relating to its intended use. No drug, particularly prescription drugs, can be called absolutely
safe. In the real life situation the drug-related risks (side effects) need to be compared with
the risk associated with the benefit to the patient to evaluate the risk-benefit ratio. It is this
ratio which one must use to judge the drug's therapeutic value. Apart from the serious side-
effect which is inherent in the drug itself, such as teratogenicity, a medicinal product can
become unsafe due to many other factors such as cross-contamination, contamination with
pathogenic organisms, very high or low potency, wrong labeling, inadequate packaging and
storage conditions. So a careful and rational evaluation should be needed.
"Daffodil International University"
Ø Potency
The product must contain adequate drug substance in its active form. Harmful degradation
products must be absent or below defined limits.
Ø Efficacy
The effectiveness of a drug indicates its biological activity in animals or in human. The active
substance should be adequately released from its dosage form.
Ø Stability
Pharmaceutical preparations may exhibit chemical or physical instability. This may result in:
(a) Reduced activity of the drug.
(b) Formation of toxic degradation products and
(c) The drug may become inelegant and thus unacceptable.
The drug substance itself and its dosage form must be sufficiently stable to retain its
minimum potency requirements satisfying the national or international pharmacopoeial
monograph. In most western countries now-a-days ±5%, beyond the labeled potency is
considered acceptable, unless the manufacturer has sound arguments for a greater variation.
The finished product must be marketed in suitable packs to ensure its stability for use up to
the expiry date when stored under specified condition.
Ø Acceptability
Acceptability refers to the consumer or market acceptability. This relates to the organoleptic
properties such as its taste, odor, color, mode of use and qualities which are not directly
noticeable to patients, e.g., too high a level of microbial contamination. A medicine should
have pharmaceutical elegance for market acceptability.
Ø Regulatory compliance
Each unit pack of the product must be clearly and correctly labeled. Moreover, the product
must fulfill the regulatory requirements. Various information in support of the product such
as potency claim, indications, side–effects, precautions, storage conditions, self-life,
manufacturing date, batch number, instructions for use etc. must comply with the drug
"Daffodil International University"
1.4 Evaluation of Tablets
Tablets are evaluated according to their physical and chemical characteristics. To monitor
tablet's quality, quantitative evaluations and assessments of chemical, physical and
bioavailability properties must be made.
1.4.1 Weight Variation
In the process of compressing a tablet, of course there are problems, one of them is the
weight variation. Usually, the range is still tolerable for large-sized tablets (diameter> 10
mm) was 3%, while for small tablet (diameter <7mm) is 5%. However, this specification
ranges vary depending on the respective industry and the active ingredient of the drug. If the
active ingredient is an extremely potent drug, in terms of the number of doses are very small
(microgram scale) has a large effect, then the range specifications for tablet weight variation
would be minimized.
Tablet weight variation in compressing process is not a trivial thing. Moreover, when
affecting the uniformity of dosage units.
v Tablet weight variation may be caused by:
Ø Distribution at Hoover caused the vibration. So, small granule pushed, large granules will
come out first, because there is a process of consolidation. Therefore, needs to be put a
uniform granule size. So, before the compressing process begins, better evaluation the
particle size distribution first.
Ø The flow of granules is not good / not free-flowing granules
Ø Particle distribution is not normal, because the specific gravity is different, so that the
Ø Keep the uniform of particle size distribution. Not too many fines and not too many
granules. Granules with a large particle diameter which causes the resultant tablet has a
variety of unsightly weight, while too fine granules which causes unsightly flow time.
Ø Lubricant or glidant less or not mixed evenly.
"Daffodil International University"
v How to overcome the weight variation of tablets:
Ø Evenly distribution of particle size
If too many fines, then need to do are create a number of more granular. This case is
commonly found on the direct compression process. This need not happen if are careful in
choosing excipient for direct compression. The problem is excipient for direct compression is
usually relatively more expensive.
If the active ingredient of the drug is stable to heat and humidity, then an easier way is to
produce by wet granulation. Through granulation, drying and sifting, which formed granules
can be more evenly. Critical points that need more attention is the moisture content and size
of mesh used at the time of sifting.
If the active ingredients of drugs are not stable to heat and humid, then try to dry granulation,
compaction with the compactor machine or slugging. Note the size of mesh used to sift.
Ø Proper use of lubricant & glidant
To solve tablet weight variation, excipient Aerosol or colloidal Silicon Dioxide can be added.
This excipient was added to the external phase. The amount used is usually 1-2% of the total
weight of the tablet. Mixing for 10-15 minutes.
Ø Specific gravity too different
This case often occurs in the manufacture of tablets that contain more than one type of
granules. Two or more active ingredients each made in separate granules (usually because of
incompatible), then at the time of compression into one, and coupled with the outer phase. Or
two granules remain separate, but when compression using two different hopper, then
compress into one tablet.
Ø Proper tooling
Proper tooling of the compression machine can solve the problem of weight variation. It
means uniform size of each punch and diameter of the compression machine and as well as
same speed in every time.
Ø Optimum machine speed
Optimum machine speed can control the weight variation of tablets because too much high or
too much slow speed can vary the weight of tablets of different station.
"Daffodil International University"
v Requirement:
Requirement is met if the weight variation of tablets is of no more than 10 tablets differs from
average weight by more than percentage given below-
Average weight of tablets
Percentage of difference
More than 324 mg
Ø Accepted tablet
Not more than two tablets are outside the percentage limit and no tablet differs by more
than two times the percentage limit according to the above table.
Ø Suspected tablet
Not more than six tablets are outside the percentage limit and no tablet differs by more
than two times the percentage limit according to the table.
Ø Rejected tablets:
One tablet differs by more than two times the percentage limit according to the table. More
than six tablets are outside the percentage limit. Tablet weight variation in compressing
process is not a trivial thing. Moreover, when affecting the uniformity of dosage units.
1.4.2 Tablet friability test
Tablet Friability Tests are constantly subjected to mechanical shocks & aberration during the
manufacturing, packing and transportation process. Such stress can lead to capping,
aberration or eve breakage of the tablets It is therefore important that the tablet is formulated
to withstand such stress. Tablet Friability In order to monitor the resistance of tablets to such
stress and to decide on their suitability for further processing such as coating, tablets are
routinely subjected to friability test.
Friability Test Minimum weight loss of the tablet should not be NMT 1%.
"Daffodil International University"
v Necessity of friability test
Ø Friability test is closely related to tablet hardness and is designed to evaluate the ability of
the tablet to withstand abrasion in packaging, handling and shipping.
Ø Hardness of the tablet can be understood.
Ø Physical stability of the tablet can be determined
1.4.3 Disintegration test
Disintegration test is widely used in the pharmaceutical industry for evaluation of
disintegration capability of formulations and quality control of different dosage forms.
Disintegration tests are performed as per the pharmacopoeial standards. Disintegration is a
measure of the quality of the oral dosage form like tablets and capsules. Each of the
pharmacopoeia like the USP, BP, IP etc each have their own set of standards and specify
disintegration tests of their own. USP, European pharmacopoeia and Japanese pharmacopoeia
have been harmonized by the International Conference on Harmonization (ICH) and are
interchangeable.
The disintegration test is performed to find out the time it takes for a solid oral dosage form
like a tablet or capsule to completely disintegrate. The time of disintegration is a measure of
the quality. This is because, for example, if the disintegration time is too high; it means that
the tablet is too highly compressed or the capsule shell gelatin is not of pharmacopoeial
quality or it may imply several other reasons. And also if the disintegration time is not
uniform in a set of samples being analyzed, it indicates batch inconsistency and lack of batch
v Disintegration Test Method
This test is provided to determine whether tablet disintegrate within the prescribed time when
placed in a liquid medium under the experimental conditions presented below. For the
purposes of this test disintegration does not imply complete dissolution of the unit or even of
its active constituent. Complete disintegration is defined as that state in which any residue of
the unit, except fragments of insoluble coating or capsule shell, remaining on the screen of
the test apparatus or adhering to the lower surface of the discs, if used, is a soft mass having
no palpably firm core.
"Daffodil International University"
v Apparatus
Ø Basket-rack assembly
The basket-rack assembly consists of six open-ended transparent tubes, each 75.0-80.0 mm
long and having an internal diameter of 20.70-23.00 mm and a wall 1.0-2.8 mm thick; the
tubes are held in a vertical position by two plates, each 88-92 mm in diameter and 5.00-8.50
mm in thickness, with six holes, each 22-26 mm in diameter, equidistant from the centre of
the plate and equally spaced from one another. Attached to the lower surface of the lower
plate is a woven stainless steel wire mesh, which has a plain square weave with 1.8-2.2 mm
apertures and with a wire diameter of 0.570-0.660 mm. The parts of the apparatus are
assembled and rigidly held by means of three bolts passing through the two plates. A suitable
means is provided to suspend the basket-rack assembly from the raising and lowering device
using a point on its axis. The design of the basket-rack assembly may be varied somewhat
provided the specifications for the glass tubes and the screen mesh size are maintained.
The use of discs is permitted only where specified or allowed. Each tube is provided with a
cylindrical disc 9.35-9.65 mm thick and 20.55-20.85 mm in diameter. The disc is made of a
suitable, transparent plastic material having a specific gravity of 1.18-1.20. Five parallel 1.9-
2.1 mm holes extend between the ends of the cylinder. One of the holes is centered on the
cylindrical axis. The other holes are centered 5.8-6.2 mm from the axis on imaginary lines
perpendicular to the axis and parallel to each other. Four identical trapezoidal-shaped planes
are cut into the wall of the cylinder, nearly perpendicular to the ends of the cylinder. The
trapezoidal shape is symmetrical; its parallel sides coincide with the ends of the cylinder and
are parallel to an imaginary line connecting the centers of two adjacent holes 6 mm from the
cylindrical axis. The parallel side of the trapezoid on the bottom of the cylinder has a length
of 1.5-1.7 mm and its bottom edges lie at a depth of 1.50-1.80 mm from the cylinder's
circumference. The parallel side of the trapezoid on the top of the cylinder has a length of
9.2-9.6 mm and its centre lies at a depth of 2.5-2.7 mm from the cylinder's circumference.
All surfaces of the disc are smooth. If the use of discs is specified, add a disc to each tube and
operate the apparatus as directed under procedure. The use of automatic detection employing
modified discs is permitted where the use of discs is specified or allowed. Such discs must
comply with the requirements of density and dimension given in this chapter.
"Daffodil International University"
v Procedure of disintegration for different tablets
The disintegration test for each dosage form is given in the pharmacopoeia. There are some
general tests for typical types of dosage forms. However, the disintegration test prescribed in
the individual monograph of a product is to be followed. If the monograph does not specify
any specific test, the general test for the specific dosage form may be employed. Some of the
types of dosage forms and their disintegration tests are:
Ø Uncoated tablets
Tested using distilled water as medium at 37+/-2 C at 29-32 cycles per minute; test is
completed after 15 minutes. It is acceptable when there is no palpable core at the end of the
cycle (for at least 5 tablets or capsules) and if the mass does not stick to the immersion disc.
Ø Coated tablets
The same test procedure is adapted but the time of operation is 30 minutes.
Ø Enteric coated/ Gastric resistant tablets
The test is carried out first in distilled water (at room temperature for 5 min.; USP and no
distilled water per BP and IP), then it is tested in 0.1 M HCL (up to 2 hours; BP) or
Stimulated gastric fluid (1 hour; USP) followed by Phosphate buffer, pH 6.8 (1 hour; BP) or
Stimulated intestinal fluid without enzymes (1 hour; USP).
Ø Chewable tablets
Exempted from disintegration test (BP and IP), 4 hours (USP). These are a few examples for
illustration. The disintegration tests for capsules, both hard and soft gelatin capsules are also
performed in a similar manner. Also, the USP also provides disintegration tests for
suppositories, peccaries etc.
v Factors affecting disintegration: several factors can significantly affect the
disintegration time of tablets:
Ø Disintegrants
A good disintegrant will quickly break up a tablet into primary particles and ensures that the
drug molecules are exposed for dissolution. Examples include corn and potato starches,
sodium starch glycolate, cellulose derivatives such as sodium carboxymethyl cellulose,
polyvinyl pyrolidone etc.
"Daffodil International University"
Ø Manufacturing process
The manufacturing processes have great influence on the disintegration behavior of tablets.
The total amount of disintegrants is added in two portions. The major part is incorporated to
the powders before granulation and the rest part is mixed with the dried granules along with
lubricants. Disintegrants added in this manner serves two purposes, those added after
granulation breaks the tablet apart into granules and the portion added before granulation
breaks the granules into fine particles.
Ø Binders and lubricants
The concentration of binder and lubricant used in the formulation has effect on disintegration
time. At lower concentration of lubricant and binder the disintegration time is lower than that
at higher concentration.
v Applications of Disintegration test:
Disintegration test is a simple test which helps in the pre-formulation stage to the formulator.
It helps in the optimization of manufacturing variables, such as compressional force and
dwell time. This test is also a simple in-process control tool to ensure uniformity from batch
to batch and among different tablets It is also an important test in the quality control of tablets
and hard gelatin capsules.
v Advantages of Disintegration tests:
1. This test is simple in concept and in practice.
2. It is very useful in pre-formulation, optimization and in quality control.
1.4.4 Dissolution Test
In the pharmaceutical industry, drug dissolution testing is routinely used to provide critical in
vitro drug release information for both quality control purposes, i.e., to assess batch-to-batch
consistency of solid oral dosage forms such as tablets, and drug development, i.e., to predict
in vivo drug release profiles. In vitro drug dissolution data generated from dissolution testing
experiments can be related to in vivo pharmacokinetic data by means of in vitro-in vivo
correlations (IVIVC). A well established predictive IVIVC model can be very helpful for
drug formulation design and post-approval manufacturing changes.
"Daffodil International University"
v Necessity of dissolution testing
Ø For selection of the formulation in the development phase:
▪ By comparison of the dissolution profiles of innovator product with those of
▪ This should be a basic strategy in R&D to maximize the chances of bioequivalence
Ø It is a requirement for comparative dissolution data for the bio-batch and innovator
batch, Same batches as used in bioequivalence study:
▪ Submit report with data, profile comparison & discussion
▪ This report forms part of pharmaceutical development report
Ø Demonstration of in vivo bioequivalence of one or more of the lower strength(s) of
an FPP may be waived based on:
▪ An acceptable in vivo BE study of the highest strength against the comparator product
▪ Demonstration of similarity of dissolution profiles,
▪ If the lower strength is proportionally similar in formula to the higher strength (bio-
▪ If all pharmacokinetic requirements are met
Ø Comparison of the release properties of pivotal batches:
▪ To demonstrate in vitro similarity of such batches.
▪ The studies should be submitted in dossier as part of the FPP development report.
Ø Selection of the dissolution specifications for product release & stability purposes:
▪ Conditions and acceptance criteria to be set
▪ The dissolution profiles of the bio-batch should be used for this purpose
▪ A dissolution specification should be able to detect inadequate release properties of the
commercial batches
Ø Post-approval amendment application
▪ Assessment of formulation changes to demonstrate that the profiles of the amendment
batch and the current batch are similar
"Daffodil International University"
v Apparatus
All parts of the apparatus, including any metal that may come into contact with the sample to
be tested or the dissolution medium, should be made from a chemically inert material and
should not adsorb, react or interfere with the preparation or the dissolution medium. The
dissolution assembly should be constructed in such a way that any vibration is reduced to a
minimum. Use an apparatus that allows full visibility of all operations.
Ø Paddle
The apparatus consists of a cylindrical vessel of suitable glass or other suitable transparent
material with a hemispherical bottom and a nominal capacity of 1000 ml. The vessel is
covered to prevent evaporation of the medium with a cover that has a central hole to
accommodate the shaft of the stirrer and other holes for the thermometer and for devices for
withdrawal of liquid. The stirrer consists of a vertical shaft with a blade at the lower end. The
blade is constructed around the shaft so that it is flush with the bottom of the shaft. When
placed inside the vessel, the shaft's axis is within 2mm of the axis of the vessel and the
bottom of the blade is 25 ± 2mm from the inner bottom of the vessel. The upper part of the
shaft is connected to a motor provided with a speed regulator so that smooth rotation of the
stirrer can be maintained without any significant wobble. The apparatus is placed in a water-
bath that maintains the dissolution medium in the vessel at 37 ± 0.5 °C.
Ø Basket
The apparatus consists of the same apparatus as described for "Paddle", except that the paddle
stirrer is replaced by a basket stirrer. The basket consists of two parts. The top part, with a
vent, is attached to the shaft. It is fitted with three spring clips, or other suitable attachments,
that allow removal of the lower part so that the preparation being examined can be placed in
the basket. These three spring clips firmly hold the lower part of the basket concentric with
the axis of the vessel during rotation. The lower detachable part of the basket is made of
welded-seam cloth, with a wire thickness of 0.254 mm diameter and with 0.381 mm square
openings, formed into a cylinder with a narrow rim of sheet metal around the top and the
bottom. If the basket is to be used with acidic media, it may be plated with a 2.5-µm layer of
gold. When placed inside the vessel, the distance between the inner bottom of the vessel and
the basket is 25 ± 2mm.
"Daffodil International University"
v Factors affecting dissolution- There are various factors that affect the dissolution
property of drugs like-
· Physicochemical factors of drug
These include the size and shape of the drug particles. From the Noyes and Whitney's
equation it is clear that-
Ø The surface area is directly related with the dissolution rate i.e., with the more surface
area (decreased particle size) the dissolution rate will also be increased.
Ø The solid phase characteristics of drugs, such as amorphicity, crystalline, states of
hydration and polymorphic structure have shown to have a significant influence on the
dissolution rate. For example, the amorphous form of novobiocin has a greater solubility
and higher dissolution rate than the crystalline form.
· Formulation factors
To satisfy certain pharmaceutical functions, various adjuncts such as diluents, binders,
disintegrants, granulating agents, lubricants, etc. are almost always used. They have very
significant effects on dissolution process e.g., usually hydrophilic lubricants like sodium
lauryl sulfate increases the dissolution rate of the drug than the hydrophobic that of
1.4.5 Assay / content uniformity test
Potency of tablet is expressed in term of grams, milligrams or micrograms (for some potent
drugs) of drugs per tablet and is given as the label strength of the product. Official compendia
or other standards provide an acceptable potency range around the label potency. For highly
potent, low dose drugs such as digitoxin, this range is usually not less than 90% and not more
than 110% of the labeled amount. For most other larger dose drugs in tablet form the official
potency range that is permitted is not less than 95% and not more than 105% of the labeled
amount. In general official potency analytical methods require that a composite sample of the
tablets be taken, ground up, mixed, and analyzed to produce an average potency value. In
composite assays, individual discrepancies can be masked by use of the blended sample.
"Daffodil International University"
v Importance of assay / content uniformity test
Ø Provide same dose to the patient.
Ø Provide optimum Plasma concentration of the drugs.
Ø Excellent output of the drug by recovering the disease.
1.5 Factors/ Source of Quality Variation
Because of the increasing complexity of modern pharmaceutical manufacture arising from a
variety of unique drugs and dosage forms, complex ethical, legal and economic
responsibilities have been placed on those concerned with manufacture of modern
pharmaceuticals. An awareness of these factors is the responsibility of all those involved in
the development, manufacture, control and marketing of quality products. A systematic
effective quality assurance program takes into consideration potential raw material, in-
process checking, packaging material, and labeling and finished product variables. The major
causes that lead to substandard drugs are given below:
(a) Addition of incorrect quantity of active ingredient or date expired sub-potent
(b) Non-uniform distribution of active ingredients and
(c) Poor stability of active ingredients in the finished product materials.
1.6 Information about the drug under analysis
1.6.1 History of Pantoprazole
Evidence emerged by the end of the 1970s that the newly discovered proton pump (H+,K+-
ATPase) in the secretory membrane of the parietal cell was the final step in acid secretion.
Literature from anaesthetic screenings led attention to the potential antiviral compound
pyridylthioacetamide which after further examination pointed the focus on an anti-secretory
compound with unknown mechanisms of action called timoprazole. Timoprazole is a
pyridylmethylsulfinyl benzimidazole and appealed due to its simple chemical structure and
its surprisingly high level of anti-secretory activity.
Optimization of substituted benzimidazoles and their antisecretory effects were studied on the
newly discovered proton pump to obtain higher pKa values of the pyridine, thereby
facilitating accumulation within the parietal cell and increasing the rate of acid-mediated
conversion to the active mediate. As a result of such optimization the first proton pump
"Daffodil International University"
inhibiting drug was released on the market, Omeprazole. Pantoprazole would follow in its
footsteps, claiming their share of a flourishing market, after their own course of development.
Pantoprazole developed by Byk Gulden (Altana subsequently purchased Byk Gulden), is
marketed around the world by a number of companies including Altana (now Nycomed),
Wyeth, and Sanofi-Aventis. Drug in Focus this month will analyse the patent landscape
surrounding Pantoprazole based on information contained in GenericsWeb's Pipeline Selector
report, with a view to launching generic equivalents.
1.6.2 Chemistry
Pantoprazole is in a group of drugs called proton pump inhibitors. Pantoprazole sodium is a
pyridinyl)methyl] sulfinyl]1H-benzimidazole sesquihydrate, a compound that inhibits gastric
acid secretion. Its empirical formula is C16H14F2N3NaO4S x 1.5 H2O, with a molecular weight
of 432.4. The structural formula is:
Figure1.2: Structure of Pantoprazole
"Daffodil International University"
Figure: 1.3-dimensional structure of Pantoprazole
Pantoprazole sodium sesquihydrate is a white to off-white crystalline powder and is racemic.
Pantoprazole has weakly basic and acidic properties. Pantoprazole sodium sesquihydrate is
freely soluble in water, very slightly soluble in phosphate buffer at pH 7.4, and practically
insoluble in n-hexane.
The stability of the compound in aqueous solution is pH-dependent. The rate of degradation
increases with decreasing pH. At ambient temperature, the degradation half-life is
approximately 2.8 hours at pH 5 and approximately 220 hours at pH 7.8.
1.6.3 Pharmacology
Pantoprazole is a widely used antiulcerent drug. Generally inactive at acidic pH of stomach,
thus it is usually given with a pro kinetic drug. It decreases the amount of acid produced in
the stomach by inhibiting H+K+ATPase (proton pumps). As it binds irreversibly to the
pumps, new pumps have to be made before acid production can be resumed. The drug's
plasma half-life is about 2 hours. Pantoprazole is used to treat erosive esophagitis (damage to
the esophagus from stomach acid), and other conditions involving excess stomach acid such
as Zollinger-Ellison syndrome. Pantoprazole is not for immediate relief of heartburn
"Daffodil International University"
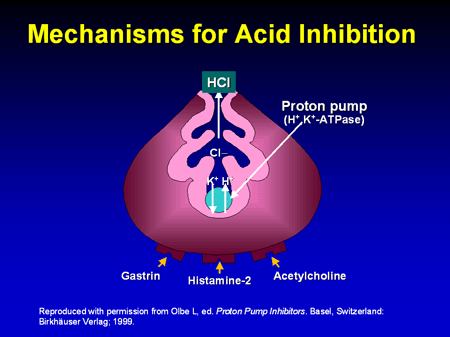
1.6.3.1 Pharmacodynamic
v Mechanism of action of Pantoprazole
Figure1.4: Mechanism for acid inhibition
• Pumps protons out of the parietal cell and potassium ions back in
• Requires energy - provided by hydrolysis of ATP to ADP, catalysed by ATPase
• The proton pump is also called H+/K+-ATPase
• Chloride ions depart through a separate ion channel
• HCl is formed in the canaliculus
• The potassium ions exit the parietal cell as countering for the chloride ions and are
then pumped back in
• A separate potassium ion channel is used for K+ ions leaving the cell
Pantoprazole is a proton pump inhibitor (PPI) that suppresses the final step in gastric acid
production by covalently binding to the (H+, K+)-ATPase enzyme system at the secretory
surface of the gastric parietal cell. This effect leads to inhibition of both basal and stimulated
gastric acid secretion, irrespective of the stimulus. The binding to the (H+, K+)-ATPase
results in a duration of antisecretory effect that persists longer than 24 hours for all doses
tested (20 mg to 120 mg).
"Daffodil International University"
v Dosage form
Pantoprazole is available in IV form and oral (tablet, suspension) form.
v Indication
Pantoprazole is indicated where suppression of acid secretion is of therapeutic benefit.
Pantoprazole is registered for the following indications: -
Ø Peptic ulcer diseases (PUD)
Ø Gastro esophageal reflux diseases (GERD)
Ø Treatment of ulcer resistant to H2 receptor antagonists (H2RAs)
Ø Treatment of ulcers induced by non-steroidal anti-inflammatory drugs (NSAIDs)
Ø Gastrointestinal (GI) bleeding from stress or acid peptic diseases
Ø Eradication of Helicobacter pylori (in combination with antibiotics)
Ø Zollinger-Ellison syndrome
Ø Prophylaxis for acid aspiration syndrome during induction of anaesthesia
v Dosage and administration
Ø Usual Adult Dose for Erosive Esophagitis
• Treatment of Erosive Esophagitis: 40 mg orally once a day for up to 8 weeks; however
an additional 8 weeks may be considered for patients who have not healed after the
initial treatment. Safety and efficacy beyond 16 weeks of therapy have not been
• Maintenance of Healing of Erosive Esophagitis: 40 mg orally once a day. Controlled
studies have been limited to 12 months of Pantoprazole therapy.
"Daffodil International University"
Ø Usual Adult Dose for Gastroesophageal Reflux Disease
• Parenteral: 40 mg once a day for 7 to 10 days, administered via intravenous infusion
over a period of 15 minutes. Intravenous therapy should be discontinued as soon as the
patient is able to resume oral therapy.
• Oral: 40 mg orally once a day, for short-term administration (up to 8 weeks); however an
additional 8 weeks may be considered for patients who have not healed after the initial
treatment. Safety and efficacy beyond 16 weeks of therapy have not been established.
Ø Usual Adult Dose for Duodenal Ulcer
Orally once a day, dose was increased every 12 weeks by 40 mg increments to a
maximum of 120 mg per day, for 28 weeks. Data have revealed that monotherapy with
daily doses of 40 mg have been associated with complete duodenal ulcer healing in up to
87% and 94% of patients after 4 weeks and 8 weeks respectively.
Ø Usual Adult Dose for Gastric Ulcer
40 mg orally once a day. Data have revealed that monotherapy with daily doses of 40 mg
have been associated with complete gastric ulcer healing in up to 87% and 97% of
patients after 4 weeks and 8 weeks respectively.
Ø Usual Adult Dose for Stress Ulcer Prophylaxis
• Stress Ulcer bleeding prophylaxis in the Critical Care Setting: 80 mg twice daily, as a
bolus infusion over a period of 15 minutes, to a maximum daily dose of 240 mg, divided
into three equal doses.
• Peptic Ulcer rebleeding prophylaxis after homeostasis in the Critical Care Setting:
80 mg IV bolus, followed by continuous infusion of 8 mg/hr for 3 days, after which
therapy may be continued with an oral PPI.
"Daffodil International University"
Ø Usual Adult Dose for Peptic Ulcer
• Stress Ulcer bleeding prophylaxis in the Critical Care Setting: 80 mg twice daily,
as a bolus infusion over a period of 15 minutes, to a maximum daily dose of 240 mg,
divided into three equal doses.
• Peptic Ulcer rebleeding prophylaxis after homeostasis in the Critical Care
Setting: 80 mg IV bolus, followed by continuous infusion of 8 mg/hr for 3 days, after
which therapy may be continued with an oral PPI.
v Side Effects
Along with its needed effects, pantoprazole may cause some unwanted effects. Although
not all of these side effects may occur, if they do occur they may need medical attention.
Ø Abdominal or stomach pain
Ø blurred vision
Ø flushed, dry skin
Ø fruit-like breath odor
Ø increased hunger
Ø increased thirst
Ø increased urination
Ø troubled breathing
Ø unexplained weight loss
v Adverse Drug Reaction
Ø Clinical Trial Experience (Adults)
Safety in nine randomized comparative US clinical trials in patients with GERD included
1,473 patients on oral Pantoprazole 20 mg or 40 mg, 299 patients on an H2-receptor
antagonist, 46 patients on another proton pump inhibitor, and 82 patients on placebo. The
most frequently occurring adverse reactions are listed in Table 1.2
"Daffodil International University"
Table 1.1: Adverse Reactions Reported in Clinical Trials of Adult Patients with GERD
at a Frequency of > 2%
Comparators
(n=1473) %
(n=345) %
Additional adverse reactions that were reported for pantoprazole in clinical trials with a
frequency of ≤ 2% are listed below by body system:
Body as a Whole: allergic reaction, pyrexia, photosensitivity reaction, facial edema
Gastrointestinal: constipation, dry mouth, hepatitis
Hematologic: leukopenia, thrombocytopenia
Metabolic/Nutritional: elevated CK (creatine kinase), generalized edema, elevated
triglycerides, and liver enzymes elevated
Musculoskeletal: myalgia Nervous: depression, vertigo
Skin and Appendages: urticaria, rash, pruritus
Special Senses: blurred vision
Ø Post marketing Experience
The following adverse reactions have been identified during post approval use of
Pantoprazole. Because these reactions are reported voluntarily from a population of uncertain
size, it is not always possible to reliably estimate their frequency or establish a causal
relationship to drug exposure.
These adverse reactions are listed below by body system:
General Disorders and Administration Conditions: asthenia, fatigue, malaise.
Hematologic: Pancytopenia, agranulocytosis.
Hepatobiliary Disorders: Hepatocellular damage leading to jaundice and hepatic failure.
Immune System Disorders: Anaphylaxis (including anaphylactic shock)
Infections and Infestations: Clostridium difficile associated diarrhea.
Investigations: Weight changes
"Daffodil International University"
Metabolism and Nutritional Disorders: Hyponatremia, hypomagnesemia.
Musculoskeletal Disorders: Rhabdomyolysis, bone fracture.
Nervous: Ageusia, ysgeusia.
Psychiatric Disorders: hallucination, confusion, insomnia, somnolence.
Renal and Urinary Disorders: Interstitial nephritis
Skin and Subcutaneous Tissue Disorders: Severe dermatologic reactions (some fatal),
including erythema multiforme, Stevens-Johnson syndrome, and toxic epidermal necrolysis
(TEN, some fatal), and angioedema (Quincke's edema).
Ø Prolonged (>1 year), high-dose therapy
• Decreased gastric acidity increases serum chromogranin A (CgA) levels and may cause
false-positive diagnostic results for neuroendocrine tumors; temporarily discontinue PPIs
before assessing CgA levels.
• PPIs may decrease the efficacy of clopidogrel by reducing the formation of the active
• Gastric atrophy reported with long-term use of another PPI.
• Therapy increases risk of Salmonella, Campylobacter, and other infections.
• Hypomagnesaemia may occur with prolonged use (>1 year); adverse effects may result,
including tetany, arrhythmias, and seizures; in 25% of cases reviewed, magnesium
supplementation alone did not improve low serum magnesium levels, and the PPI had to
be discontinued.
• Infusion related reactions including thrombophlebitis and hypersensitivity reported.
• Prolonged treatment may lead to vitamin B12 malabsorption.
• Relief of symptoms does not eliminate the possibility of a gastric malignancy.
• Relief of symptoms does not preclude the presence of a gastric malignancy. Risk of
salmonella and campylobacter infections increased with use of proton pump inhibitors.
v Special warnings and precautions for use
Ø Pantoprazole for Delayed-Release Oral Suspension and Pantoprazole for Delayed-Release
Tablets should not be split, crushed, or chewed.
Ø Pantoprazole oral suspension packet is a fixed dose and cannot be divided to make a
"Daffodil International University"
Ø Pantoprazole Delayed-Release Tablets should be swallowed whole, with or without food
Ø Concomitant administration of antacids does not affect the absorption of Pantoprazole
Delayed-Release Tablets.
Ø Pantoprazole for Delayed-Release Oral Suspension should be administered approximately
30 minutes before a meal.
Ø Pantoprazole for Delayed-Release Oral Suspension should only be administered in apple
juice or applesauce, not in water, other liquids, or foods.
Ø Immediately report and seek care for any cardiovascular or neurological symptoms
including palpitation, dizziness, seizures, and tetany as these may be signs of
hypomagnesaemia.
Ø Immediately report and seek care for diarrhea that does not improve. This may be
a sign of Clostridium difficile associated diarrhea.
v Contra-indications
Pantoprazole is contraindicated in patients with known hypersensitivity to any component of
the formulation.
v Drug Interactions
Ø Interference With Antiretroviral Therapy
Concomitant use of atazanavir or nelfinavir with proton pump inhibitors is not
recommended. Coadministration of atazanavir or nelfinavir with proton pump inhibitors is
expected to substantially decrease atazanavir or nelfinavir plasma concentrations and may
result in a loss of therapeutic effect and development of drug resistance.
Ø Coumarin Anticoagulants
There have been postmarketing reports of increased INR and prothrombin time in patients
receiving proton pump inhibitors, including Panyoprazole, and warfarin concomitantly.
Increases in INR and prothrombin time may lead to abnormal bleeding and even death.
Patients treated with proton pump inhibitors and warfarin concomitantly should be
monitored for increases in INR and prothrombin time.
"Daffodil International University"
Ø Clopidogrel
Concomitant administration of pantoprazole and clopidogrel in healthy subjects had no
clinically important effect on exposure to the active metabolite of clopidogrel or
clopidogrelinduced plateletinhibition. No dose adjustment of clopidogrel is necessary when
administered with an approved dose of Pantoprazole.
Ø Drugs For Which Gastric pH Can Affect Bioavailability
Pantoprazole causes long-lasting inhibition of gastric acid secretion. Therefore, pantoprazole
may interfere with absorption of drugs where gastric pH is an important determinant of their
bioavailability (e.g., ketoconazole, ampicillin esters, and iron salts).
Ø Methotrexate
Case reports, published population pharmacokinetic studies, and retrospective analyses
suggest that concomitant administration of PPIs and methotrexate (primarily at high dose)
may elevate and prolong serum levels of methotrexate and/or its metabolite
hydroxymethotrexate. However, no formal drug interaction studies of Methotrexate with
PPIs have been conducted.
v Pregnancy and lactation
Ø Pantoprazole Pregnancy Warnings
Pantoprazole has been assigned to pregnancy category B by the FDA. Animal data have
failed to reveal evidence of fetal harm after rats or rabbits were given doses 88 and 40 times
the recommended human dose (based on body surface area), respectively. There are no data
from controlled human studies. Pantoprazole should only be used during pregnancy when
need has been clearly established.
Ø Pantoprazole Breastfeeding Warnings
Pantoprazole and its metabolites are excreted in the milk of rats. Pantoprazole has been
detected in human milk in a study of a single nursing mother following a single 40 mg oral
dose. Since many drugs are excreted in human milk, the manufacturer recommends that due
to the potential for serious adverse reactions in nursing infants, a decision should be made to
discontinue nursing or discontinue the drug, taking into account the importance of the drug
"Daffodil International University"
v Storage conditions
Store Pantoprazole for Delayed-Release Oral Suspension and Pantoprazole Delayed-Release
Tablets at 20° to 25°C (68° to 77°F); excursions permitted to 15° to 30°C (59° to 86°F).
v Shelf life
Shelf life of pantoprazole is 3 years but after first opening of the bottle use the medicinal
product within three months.
1.6.3.2 Pharmacokinetic
Pantoprazole Delayed-Release Tablets are prepared as enteric-coated tablets so that
absorption of pantoprazole begins only after the tablet leaves the stomach. Peak serum
concentration (Cmax) and area under the serum concentration time curve (AUC) increase in a
manner proportional to oral and intravenous doses from 10 mg to 80 mg. Pantoprazole does
not accumulate, and its pharmacokinetics are unaltered with multiple daily dosing. Following
oral or intravenous administration, the serum concentration of pantoprazole declines
biexponentially, with a terminal elimination half-life of approximately one hour.
In extensive metabolizers with normal liver function receiving an oral dose of the enteric
coated 40 mg pantoprazole tablet, the peak concentration (Cmax) is 2.5 µg/mL; the time to
reach the peak concentration (tmax) is 2.5 h, and the mean total area under the plasma
concentration versus time curve (AUC) is 4.8 µg•h/mL (range 1.4 to 13.3 µgh/mL).
Following intravenous administration of Pantoprazole to extensive metabolizers, its total
clearance is 7.6-14.0 L/h, and its apparent volume of distribution is 11.0-23.6 L.
A single oral dose of Pantoprazole for Delayed-Release Oral Suspension, 40 mg, was shown
to be bioequivalent when administered to healthy subjects (N = 22) as granules sprinkled over
a teaspoonful of applesauce, as granules mixed with apple juice, or mixed with apple juice
followed by administration through a nasogastric tube.
v Absorption
After administration of a single or multiple oral 40 mg doses of Pantoprazole Delayed-
Release Tablets, the peak plasma concentration of pantoprazole was achieved in
approximately 2.5 hours, and Cmax was 2.5 µg/mL. Pantoprazole undergoes little first-pass
metabolism, resulting in an absolute bioavailability of approximately 77%. Pantoprazole
absorption is not affected by concomitant administration of antacids.
"Daffodil International University"
Administration of Pantoprazole Delayed-Release Tablets with food may delay its absorption
up to 2 hours or longer; however, the Cmax and the extent of pantoprazole absorption (AUC)
are not altered. Thus, Pantoprazole Delayed-Release Tablets may be taken without regard to
timing of meals.
Administration of Pantoprazole granules 40 mg with a high-fat meal delayed median time to
peak plasma concentration by 2 hours. With a concomitant high-fat meal the Cmax and AUC
of Pantoprazole granules 40 mg sprinkled on applesauce decreased by 51% and 29%
respectively. Thus, Pantoprazole for Delayed-Release Oral Suspension should be taken
approximately 30 minutes before a meal.
v Distribution
The apparent volume of distribution of Pantoprazole is approximately 11.0-23.6 L
distributing mainly in extracellular fluid. The serum protein binding of pantoprazole is about
98% primarily to albumin.
v Metabolism
Pantoprazole is extensively metabolized in the liver through the cytochrome P450 (CYP)
system. Pantoprazole metabolism is independent of the route of administration (intravenous
or oral). The main metabolic pathway is demethylation by CYP2C19 with subsequent
sulfation. Other metabolic pathways include oxidation by CYP3A4. There is no evidence that
any of the pantoprazole metabolites have significant pharmacologic activity.
v Elimination
After a single oral or intravenous dose of 14C-labeled pantoprazole to healthy, normal
metabolizer volunteers approximately 71% of the dose was excreted in the urine, with 18%
excreted in the feces through biliary excretion. There was no renal excretion of unchanged
"Daffodil International University"
1.6.4 Some market preparation available in Bangladesh
Name of the company
Brand name
Dosage form available
Inj: 40 mg / ampoule
Inj: 40 mg / ampoule
Inj: 40 mg / ampoule
Inj: 40 mg / ampoule
"Daffodil International University"
Chapter Two
Materials & Methods
"Daffodil International University"
2.1 Materials
2.1.1. Collection of Sample
There are many brands of Pantoprazole tablets in Bangladesh. Samples were collected from
retail medicine shop of different areas of Dhaka city. The samples were properly checked for
their physical appearance, name of the manufacturer, batch number, manufacturing data,
expiry date, manufacturing license number, D.A.R. number and maximum retail price at the
time of purchase. No samples were bought and analyzed whom date of expiry had already
been passed. Collected samples also covered small, medium and big companies. The samples
were then coded with ethics for analysis.
2.1.2 Collection of standard
The U.S.P reference standard of Pantoprazole was obtained from Incepta Pharmaceutical Ltd.
The purity of the reference standard was 99.9%.
2.1.3 Coding of Tablet
Pantoprazole tablet from 5 different pharmaceutical companies were coded as
2.1.4 Labeling on the Inner Carton of the collected samples
Each of the containers of tablets and injection labeled with the following particulars:
(a) Brand name of the product
(b) Name of the manufacturer
(c) Composition of the product
(d) Batch number
(e) Manufacturing date
(g) Manufacturing license number
(h) D.A.R. number
(i) Maximum retail price (M.R.P.)
"Daffodil International University"
2.1.5 Reagents
Ø Distilled water
Ø Standard Pantoprazole
Ø Sodium hydroxide
Ø Phosphate buffer:
(a) 0.2 M Potassium Dihydrogen Phosphate
(b) 0.1 N Sodium Hydroxide
(c) Phosphoric acid
2.1.6 Instruments
Table No.2.1: Instruments used in this study
Instruments
Electronic balance
Ohaus CP213, China
Friability test apparatus
Disintegration test apparatus
Dissolution test apparatus USP Minhua,China
UV Visible Spectrophotometer PG instrumentation,England
2.2 Methods
2.2.1 Physical Analysis
2.2.1.1 Weight Variation test
The weight variation is routinely measured to help ensured that a tablet contains proper
"Daffodil International University"

Ø Procedure
10 tablets were taken and weighed individually by an analytical balance. The average weight
of the tablets was calculated. Then % of weight variation is calculated by using the following
Individual weight – average weight
% of weight variation = ´ ´ 100
In this way the weight variation for 5 different brands of Pantoprazole tablets were measured
and the observed value for each sample was recorded.
Figure 2.1: Analytical balance used for weight variation test
2.2.1.2 Friability test of tablets
Tablets friability results in weight loss of tablets in the package container, owing to partial
powdering, chipping or fragmentation of the tablets on attrition or wear. Tablets that are
chipped or mechanically eroded and no longer have sharp edges are of reduced
pharmaceutical elegance and reduced quality. Tablet friability often reflects lack of
cohesiveness on compression of the dry granulation from which the tablets are made.
Ø Procedure
5 tablets were taken and weighed by an analytical balance. Then the tablets were put in
Friabilator and the machine is allowed rotate at 30 R.P.M for 4 minutes .The tablets were
weighed again. Then the % of friability was calculated by the following formula:
Initial weight – final weight
% of friability = ´ 100
"Daffodil International University"

In this way % of friability was determined for 5 different brands of tablets and the observed
result for each sample was recorded.
Figure 2.2: Friability test apparatus used for friability test
2.2.1.3 Disintegration time test of tablets
Disintegration time is the length of time required for causing disintegration of tablet. This test
is important to evaluate a tablet since it directly influences the onset of action. This test not
only evaluates the quality but also the bioavailability and effectiveness of tablets.
Ø Procedure:
As Pantoprazole is an enteric coated tablet so at first the disintegrity was observed in 0.1N
HCl for 2 hours then check the disintegration time in phosphate buffer. About 700ml
Phosphate buffer was taken in 1000ml beaker and the beaker was placed into the device. One
Pantoprazole tablet was placed in each tube of basket rack & plastic disk is placed over each
tablet & the basket rack is accurately positioned into the beaker. The temperature was
maintained as 37±5
. A motor driven device helps to move the basket up down through a
distance of 5-6cm at a rate of 28-32 cycles per minutes.The time at which all the
Pantoprazole tablets passed through the sieve was the disintegration time & the average
disintegration time were calculated. In this way disintegration time was determined for 5
different brands of Pantoprazole tablets and the observed result for each sample was
"Daffodil International University"

Figure 2.3: Disintegration apparatus used for DT test
2.2.1.4 Dissolution rate test of tablets
Dissolution is the property or tendency of a drug to undergo solution, which affects the rate
of drug absorption.
Ø Medium: Phosphate Buffer pH 6.8
Ø Procedure
900 ml of 0.1N HCl solution was filled into 1000ml beaker of dissolution apparatus. Each
Pantoprazole tablets of each brand were placed into each beaker. The test was repeated for 3
times for 3 samples of each brand. The dissolution medium was heated up to 37±o.5 by an
auto heater & 100 R.P.M was adjusted. 5 ml solution was withdrawn from beaker after 2
hours and fill with 5 ml distill water. Then withdrawn solution was filtered through filter
paper. The withdrawn solution of the sample absorbance was measured at 289 nm by using
UV-visible spectrophotometer. Finally the percent release of Pantoprazole tablet was
determined. As Pantoprazole tablets were enteric coated it would not release not more than
10% after 2 hrs treatment with gastric HCl. Its dissolution medium is phosphate buffer. So
after that phosphate buffer of 6.8 pH was prepared and filled the beaker with pouring 900 ml
buffer in each beaker. The treated Pantoprazole tablets with 0.1N HCL for two hours in
dissolution apparatus was placed in the phosphate buffer dissolution medium. 5 ml sample
was withdrawn in every 10 minutes interval for 4 times and filled the beaker by adding 5
ml of distill water. Then the sample was filtered and by performing 10 times dilution
absorbance was measured at 289 nm.
"Daffodil International University"
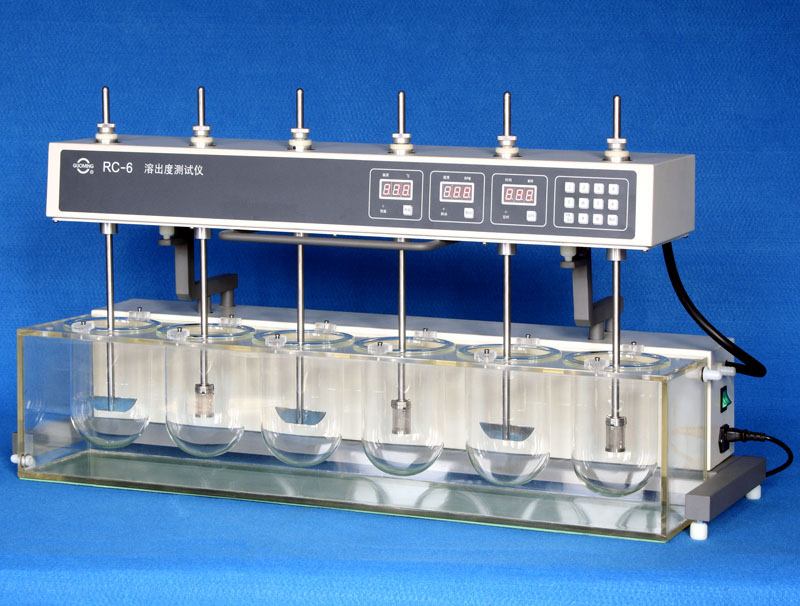
In this way the dissolution rates of 5 different brands of Pantoprazole tablets were
determined and the observed value for each sample was recorded.
Figure 2.4: dissolution apparatus used for dissolution test
2.2.2 Chemical Analysis
2.2.2.1 Preparation of standard curve of Pantoprazole
10 mg of Pantoprazole was measured by the electronic balance and placed in 100ml
volumetric flask and dissolved by ethanol. Then the concentration of solution was attained
100µg/ml by adding Phosphate buffer. Then A series of standard solution of standard
pantoprazole eg, 2µg/ml, 4µg/ml, 6µg/ml, 8µg/ml, 10µg/ml, 12µg/ml, 14µg/ml, 16µg/ml,
were taken for check Absorbance at 242 nm against a blank for each solution by UV-
spectrophotometer. The measured absorbances were plotted against the respective
concentration of the standard solutions which give a straight line.
"Daffodil International University"
Table 2.2: Absorbance of different concentration of standard Pantoprazole solution
measured at 271 nm
Concentration (mcg /ml)
Absorbance
Average absorbance
"Daffodil International University"

Figure: 2.5 Standard curve of Pantoprazole
Figure 2.6: UV spectroscopy used for measuring the absorbance
"Daffodil International University"
2.2.2 Assay / potency test
2.2.2.1 Preparation of standard solution
Ø Preparation of standard solution of Pantoprazole:
To prepare a standard solution,10 mg of Pantoprazole was measured by the electronic balance
and placed in 100 ml volumetric flask and dissolver by ethanol. Then the concentration of
solution was attained 100µg/ml by adding Phosphate buffer. Then 1 ml solution was taken
and diluted to 10 ml and the concentration goes to 10 mcg /ml.
Ø Preparation of assay solution
20 tablets of of each brands of Pantoprazole were weighed and powdered. Equivalent weight
of 10 mg of Pantoprazole sodium was weighed as sample and dissolved in 100 ml ethanol
then the solution was filtered. 1 ml of sample was taken and made the volume 10 ml.
Absorbance was measured at 289 nm using UV Spectrophotometer.
Ø Measurement
The absorbance of both standard and sample were measured at 289 nm using UV
Spectrophotometer.
Ø Calculation
Finally the assay was calculated by using the following equation.
Assay of sample = ´ DF ´ P ´ Wt Avg
Abs of Std ´ Wt of Sam
Where, Abs of Sam = Absorbance of sample
Wt of Std = Weight of standard
Abs of Std = Absorbance of standard
Wt of Sam = Weight of sample
DF = Dilution factor
P = Potency of standard
Wt Avg = Average weight of sample
"Daffodil International University"
Chapter Three
Results & Discussion
"Daffodil International University"
3.1 Weight Variation
The weight variations of five brands Pantoprazole were determined & the observed results
are shown in the following table. The USP specification of weight variation: ±7.5 for 130 to
324mg average weight of tablet & ± 5% for more than 324mg of average weight of tablet. It
was observed that all of the brands meet the USP specification.
Table 3.1: Weight Variation of P01
SI
Individual
No weight(mg)
Weight(mg)
variation %
Table 3.2 : Weight variation of P02
Individual
RSD% Comment
No weight(mg)
Weight(mg)
variation %
"Daffodil International University"
Table 3.3: Weight variation of P03
Individual
No weight(mg)
Weight(mg)
variation %
Table 3.4: Weight Variation of P04
Individual
No weight(mg)
Weight(mg)
variation %
Table 3.5: Weight variation of P05
Individual
No weight(mg)
Weight(mg)
variation %
"Daffodil International University"
3.2 Friability
The friability of 5 different brands of Pantoprazole tablets were measured according to the
procedure and the observed results are shown in the table 3.2
Table 3.2: Friability of various brands of pantoprazole tablets
Sample code
initial Total
final Observed
tablets taken
weight (mg)
weight (mg)
friability
f fria
o 0.15
Sample Code
Figure: 3.1 Friability of various brands of Pantoprazole tablets.
"Daffodil International University"
USP Specification for Friability of tablets:
Allowed range =1.0%
From the above results (Table 3.2) it is appeared that all brands of Pantoprazole tablets
complied with the BP / USP specification of friability.
3.3 Disintegration time
The disintegration time of five brands in phosphate buffer of pantoprazole are shown in table.
Before checking the disintegration in phosphate buffer they were treated in o.1 N HCl for 2
hours but no disintegration occur because of its enteric coating. The specification of
disintegration time is 5 to 30 minutes.
Table 3.3: Disintegration time of 5 brands of Pantoprazole tablets
T1(min) T2(min) T3(min) T4(min) T5(min) T6(min) Avg.
"Daffodil International University"
Disintegration time
Sample Code
Figure 3.2.: Disintegration time of various brands of Pantoprazole tablets.
It was seen from result (table) that none of the marketed Pantoprazole sample exceeded the
specification and therefore it can be said that e entire marketed sample complied with the
specification for tablet disintegration time.
3.4 Assay & Potency test
6 tablets of of each brands of pantoprazole were weighed and powdered. 10 mg of
pantoprazole sodium equivalent to pantoprazole was weighed and dissolved in 100 ml
ethanol then the solution was filtered. 1 ml of sample was taken and made the volume 10 ml.
Absorbance was measured at 289 nm using Shimadzu UV Spectrophotometer. Finally the
assay was calculated by using the following equation.
Absorbance of sample ´ Weight of standard
Assay of sample = ´ DF ´ Potency ´ Wt Avg
Absorbance of standard ´ Weight of sample
Where, Abs of Sam = Absorbance of sample
Wt of Std = Weight of standard
Abs of Std = Absorbance of standard
Wt of Sam = Weight of sample
DF = Dilution factor
P = Potency of standard
Wt Avg = Average weight of sample
"Daffodil International University"
Table: 3.4 Potency of P01
Abs of Wt of
Potency SD
Average potency = 98.14%
All the tablets meet the USP specification of potency
Table: 3.5 Potency of P02
Abs of Wt of
Potency SD
Average potency = 97.04%
All tablets meet the USP specification of potency
"Daffodil International University"
Table: 3.6 Potency of P03
Abs of Wt of
Potency SD
Average potency = 97.51%
All the tablets meet the specification of USP guideline of potency
Table: 3.7 Potency of P04
Abs of Wt of
Potency SD
Average potency = 95.76%
Two tablets don't meet the specification of the USP guidelines of potency.
"Daffodil International University"
Table: 3.8 Potency of P05
Abs of Wt of
Potency SD
Average potency = 94.60%
Two tablets do not meet the specification of the USP guidelines of potency.
Sample code
Figure 3.3: Average potency of different market preparations of Pantoprazole tablet.
"Daffodil International University"
3.5 Dissolution test
The dissolution rate of five brands of Pantoprazole tablets was determined. The observed
results were shown in table. The drug release% was plotted against the times, which give
dissolution curve.
USP specification: Not more than 10% in 0.1 N HCl in 2 hours & not less than 75% of the
labeled amount of pantoprazole to be dissolved in 45 minutes. the remaining Five Brands of
pantoprazole tablets meet the specification.
Table 3.9 Dissolution rate after 2 hrs. in 0.1 N HCl
Sample % of drug release
% o release 4
Sample Code
"Daffodil International University"
Figure 3.4 : Dissolution rate of different brands of Pantoprazole in 0.1N HCl after 2
Table 3.10 Dissolution rate of P01 in phosphate buffer
SI Time interval drug release %
Average % SD
Table 3.11 Dissolution rate of P02 in phosphate buffer
SI Time interval drug release %
Average % SD
Table 3.12 Dissolution rate of P03 in phosphate buffer
SI Time interval drug release %
Average % SD
"Daffodil International University"
Table 3.13 Dissolution rate of P04 in phosphate buffer
SI Time interval drug release %
Average % SD
Table 3.13 Dissolution rate of P05
SI Time interval drug release %
Average % SD
"Daffodil International University"
Figure 3.5: Dissolution Rate of Various Brands of Pantoprazole Tablets
All the brands meet the specification of the USP standard as they did not release more than
10% drug in 0.1 N HCL and all brands more than 75% within 45 minutes.
"Daffodil International University"
v Conclusion
Pantoprazole tablets have been analyzed to find their correct quality status. For this purpose,
the marketed sample of five brands of Pantoprazole tablets was analyzed by using established
methods and apparatus. RSD value of weight variation of different drugs is in the range of
(0.78-1.65)%, maximum friability of the tablets of all brands is 0.4%, maximum
disintegration time in phosphate buffer is 17.42 minutes, Maximum average potency among
the brands is 98.14 % and minimum potency is 94.60%, among the brands the maximum drug
release in 0.1N HCl is 6.06 , minimum dissolution in phosphate buffer after 45 minutes is
79.94% and the maximum is 88.03%. The result of friability, weight variation, and assay and
disintegration & dissolution tests of all marketed products comply with pharmacopoeal limit
except some parameter in case of lower listed company. The present study, although
performed on a limited scale yet on the basis of professional judgment the data reported in
this study can help the Drug Control Authority to get an idea about the quality status of the
marketed Pantoprazole preparations in Bangladesh.
"Daffodil International University"
v References
Ø "The theory and practice of industrial pharmacy", Lachman, Liberman, Kanig, third
Ø Gupta A. K. ,2001. "Introduction of Pharmaceutics-1" 3rd edition ,CBS Publishers &
Distributors, New Delhi, India. P.240-271
Ø Khan Dr.M.Shah Nawaz, "Assurance of Quality Pharmaceuticals", 1990, pp 33 – 35.
Ø British Pharmacopoeia 2009
Ø The United States Pharmacopea 2009
Ø Edwin K Jackson; Goodman & Gilman's 2007 ; PPI ; The Pharmacological Basis of
Ø 2007; Lippincott's Illustrated Reviews: Pharmacology; 5th Edition, Lippincott's-Raven
publishers NY; P 226-233
Ø Simler, R., Walsh, G., Mattaliano, R.J., Guziewicz, N., and Perez-Ramirez, B. (2008).
Maximizing Data Collection and Analysis During Preformulation of Biotherapeutic
Proteins. BioProcess International 6(10), 38-45.
Ø M. Nocent, L. Bertocchi, F. Espitalier, M. Baron and G. Couarraze. (2001). Definition of
a solvent system for spherical crystallization of salbutamol sulfate by quasi-emulsion
solvent diffusion (QESD) method. Journal of Pharmaceutical Sciences 90 (10), 1620-
Ø Patrick, G (2009). An Introduction to Medicinal Chemistry. 4th ed. USA: Oxford
University Press. p172.
Ø http://www.medscape.com/viewarticle/406969_2
Ø http://en.wikipedia.org/wiki/Pantoprazole
Ø http://www.drugbank.ca/drugs/DB00213
Ø http://www.ncbi.nlm.nih.gov/pubmed/8930575
"Daffodil International University"
Ø Kalakuntla, R., Veerlapati, U., Chepuri, M., Raparla, R. (2010). Effect of various super
disintegrants on hardness, disinte-gration and dissolution of drug from dosage form. J.
Adv. Sci. Res, 1(1): 15-19.
Ø Fitton and L. Wiseman. Pantoprazole: a review of its pharmacological
properties and therapeutic use in acid-related disorders Drugs 51(3): 460–82 (1996)
Ø P. W. Jungnickel. Pantoprazole: a new proton pump inhibitor. Clin. Ther. 22 (11): 1268–
Ø Journal of Chemical and Pharmaceutical Research
Ø Development of UV Spectrophotometric method for estimation of Pantoprazole in
pharmaceutical dosage forms Rajnish Kumar*, Harinder Singh and Pinderjit Singh
Department of Health and Family Welfare, State Food, Drug and Excise Laboratory,
Punjab,Chandigarh, India
Ø RB Kakde, SM Gedam, NK Chaudhary, AG Barsagade, DL Kale, AV Kasture.
Ø Journal of Pharm Tech Research.2009; 1(2): 386-389.
Ø K Basavaiah, UR Anil Kumar, Indian Journal of Chemical Technology. 2007; 14:611-
Ø AA Syed, A Syeda. Bull. Chem. Soc. Ethiop. 2007; 21(3): 315-321
Ø R Kalaichelvi, MF Rose, K Vedival, E Jayachandran. International Journal of Chemistry
Research. 2010; 1(1): 6-8
"Daffodil International University"
Source: http://dspace.library.daffodilvarsity.edu.bd:8080/bitstream/handle/123456789/1105/Project%20Report.pdf%20AV4628.pdf?sequence=1
alturos.co.uk
© Alturos Ltd 2006-2013 Applying Process Improvement in an NHS Pharmacy to Reduce Cost, Increase Productivity and Create a Better Working Environment In July 2010, the Pharmacy at one site of one of London's largest NHS Foundation Trust's, started applying Lean working methods. Since that time, a number of projects have developed. This article
Lengley
Fibromyalgie : la (Grande-Bretagne) Pour ses lecteurs, Myalgies pouvoirs publics anglais, annonça "la est sur tous les fronts fibromyalgie est maintenant une entité avec ses correspondants. reconnue et les médecins qui persistent à Kathy Longley, journaliste dire que ce n'est qu'une vue de l'esprit, se anglaise au Family Magazine, moquent ironiquement de leur propre igno-