Bvcaa.org

POLICY & PROCEDURE
TITLE: ALLERGY SHOTS (IMMUNOTHERAPY)
Scope/Purpose: To provide guidelines to ensure safe administration of immunotherapy agents.
Division/Department: All Clinics
Policy/Procedure #:
Original Date: March 11, 2016
_X New _Replacement for:
Date Reviewed:
Date Revised:
CPIC Approved:
Board Approved:
Responsible Party:
Antigen: A molecule recognized as ‘foreign' by the body's immune system, causing the release of antibodies.
Allergens: A type of antigen that produces an abnormal vigorous immune response in which the immune
system fights off a perceived threat that would otherwise be harmless to the body.
Allergic reaction: The body's response to allergens. Severity of reaction can range from mild symptoms to life
threatening emergency. Allergic reactions can be caused by the following types of contact and include the
following symptoms:
Ingestion (Consuming food or drink one is sensitive to. Foods commonly associated with allergies are
peanuts, cow's milk, eggs, wheat, soy, nuts, sesame, fish, shellfish and preservatives such as sulphites.) Reaction symptoms can include digestive problems, hives or trouble breathing.
Inhalation (Breathing in pollen, dust mite droppings, perfume or pet dander). Reaction symptoms can
be watery eyes, congestion and breathing difficulty.
Direct (Brushing against an allergy causing plant such as poison ivy, poison oak, etc., contact with
insect venom - wasps, fire ants, bees, or contact with latex). Reaction symptoms can range from skin irritation and local swelling to life-threatening shock.
Medication administration (Penicillin/other antibiotics). Reaction symptoms can be hives, rash, fever
and difficulty breathing. Severe reactions can be life threatening.
Anaphylaxis (also called Systemic Reaction): An extremely serious form of allergic reaction which causes
multiple symptoms throughout the body. Symptoms include rash, hives, nausea and vomiting, difficulty
breathing, dangerous drop in blood pressure, swelling of eyes and throat and possibly, shock. Implement 911.
Notify allergist.
Immunotherapy: Allergen immunotherapy, also known as desensitization, is a medical treatment for
environmental allergies, allergies to insect bites and asthma. Immunotherapy:
Involves exposing people to larger and larger amounts of allergen in an attempt to
Change the immune system's response.
Consists of protocols that generally involve weekly injections during a ‘build-up' phase that can last
several months to a year, followed by monthly maintenance injections for a period of 3-5 years.
Localized Reaction: Minor swelling, redness or itching at the injection site after administration of the allergy
shot. Dime to quarter size swelling at the injection site is normal within minutes of the injection. Larger local
reactions (great than quarter size) can occur over 24 hours and last 2-3 days. Larger local reactions require
allergist notification and dosage adjustment.
POLICY:
Patients receiving immunotherapy injections (allergy shots) must be under the care of an allergist.
PROCEDURE:
1. Initiation of care – necessary referral components:
A. Referral - The patient's allergist must provide the following:
Prescribing physician's name, address, phone and fax numbers
Allergy Injection Protocol with patient's signed consent
Allergen extract for injection
B. Protocols - must contain specific instructions on:
Dosing, contents and strengths of extracts
Dose adjustments for missed shots, late shots or reactions
Escalation and maintenance dosing, interval between injections
Missed dose schedule
Use of new vials
Seasonal exposures
Reaction to last dose administered
C. Clarification MUST occur should orders (Protocol) be unclear or incomplete. The Provider or clinic
nurse are responsible for performing clarification.
2. Initial and Subsequent Visits:
A. Medicare and Medicaid patients require injections be administered in provider visits.
Vital Signs must be recorded for all ‘Provider' visits.
A minimum of temperature must be performed for ‘Nurse Only' visits.
B. Confirm:
A PROVIDER IS IN THE BUILDING – No provider present – No Shot!
The patient has EpiPen – No EpiPen – No Shot!
The patient has taken antihistamine at prior night or in a.m. – No Antihistamine – No Shot!
The patient is NOT ill – Ill with any of the following – No Shot!
o Shortness of Breath (If Asthmatic and used inhaler in LAST 24 HOURS)
o Productive Cough or colored nasal drainage
o Hives
o Had an insect sting in the last 24 hours
o Fever – Temperature above 99°F - Injection possible - consult provider
The patient is NOT taking Beta-Blockers. Beta-Blockers or MAOIs – No Shot!
o Ask the patient if the patient is taking any new medications
o Consult with provider regarding the patient's medications: Allergy injections are
contraindicated for patients taking Beta-Blockers (Coreg/Carvedilol, Lopressor/Metaprolol, Tenormin/Atenolol, Corgard/Nadolol, Inderal/Propanolol) or Monamine Oxidase Inhibitors - M.A.I.O's – (Marplan/Isocarboxid, Nardil/Phenelzine, Emsam/Selegiline, Parnate/Tranlcypromine.
C. Use Caution:
CONSULT PROVIDER if patient has started new medications.
Allergy Injections are contraindicated for persons taking BETA-BLOCKERS or MONOAMINE OXIDASE INHIBITORS (M.A.O.I.'S.)
REVIEW PROTOCOL FOR NEW VIAL: Some Allergists require patients to receive 1st
injection of new vial at the Allergists office.
QUESTION THE PATIENT REGARDING RESPONSE TO LAST ALLERGY SHOT
REMEMBER: Localized reaction with redness greater equal to or greater than 3 cm
(quarter size) or SIGNIFICANT DISCOMFORT at time of injection or delayed (within 24
hours of injection), require protocol review of dosing instructions and allergist notification.
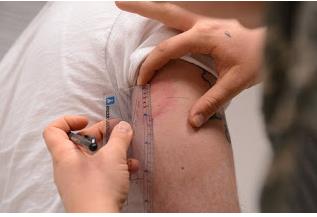
3. Administration:
A. Inform patient of procedure. Have patient be seated. If patient has a history of fainting from
injections, have patient lie on exam table.
B. Injections are given subcutaneously using a 1 mL syringe with a 26 or 27 gauge needle.
Injections should be given in the posterior portion of the middle third of the upper arm at
the junction of the deltoid and triceps muscle.
Syringe should be aspirated to check for blood return in syringe prior to injecting. If
blood is present, DO NOT inject. Use a different needle and a different site.
4. Documentation:
A. Document the injections on the ‘Allergy Flow Sheet'
B. Complete BVCAA: Nurse Visit – Allergy Injections
C. If more than one injection is given, do the following:
In ‘Treatment' section of progress note – select treatment and go to ‘Procedures'
In section labeled ‘Today's orders' – delete ‘one injection' (Select "Yes" to question "Are
you sure you want to delete one injection?)
Proceed to ‘look up' and type in ‘inj' – then select ‘MULTIPLE INJECTIONS' and close
Proceed to Billing information' and select ‘Procedure Codes'
o Select Procedure Code 95117 for multiple injections o Change number of units to reflect number of injections administered
5. Post Administration Observation and Documentation:
A. Observe the patient for 20" (minutes) after the injection. The onset of anaphylactic shock occurs
within 30" (minutes) following injection.
B. Measure injection site for inflammation, swelling, wheal and flare size in longest diameter.
Localized reaction with redness greater equal to or greater than 3 cm (quarter size)
requires protocol review
Notify allergist
Follow protocol directions regarding dosing
C. Remind patients they should NOT EXERCISE for at least 2 hours after injection.
D. Document reaction on the Allergy Flow Sheet.
6. Reactions and Treatment:
A. Localized Reaction – Minor swelling or redness at site.
Report to allergist as allergist may wish to consider adjustment of future doses
Topical steroids may be applied for reactions greater than 2" (inches)
B. Systemic Reaction: A ‘911' Medical Emergency (AIRWAY – BREATHING - CIRCULATION)
Administer EpiPen near the allergy shot injection site
Call provider
Activate 911
Administer Oxygen via mask
STAY WITH PATIENT
Place patient in supine position (flat) with feet elevated
Administer Benadryl from EMERGENCY BOX as directed by the provider
Administer Albuterol via nebulizer if Provider orders
Monitor and record Vital Signs, including Pulse Oximetry readings
Ensure Documentation is Complete in Medical Record
Complete Occurrence Variance Report
Notify the patient's allergist of injection response
Storage:
A. Allergy shot extract MUST be clearly labeled with:
Patient's name
Contents/Name of the vial
Expiration Date
B. Refrigerator temperature should be maintained between 37.4°F and 42.8°F. If the extract is
exposed to heat due to refrigerator malfunction or power failure, or frozen:
Contact the referring Allergist for instructions.
Document allergist's instruction in the progress note.
C. Allergy Extract must be ‘in date'.
Clinic staff and patient share the responsibility to notify allergist replacement extract is
needed at least 3 weeks prior to expiration date on vial.
The patient is responsible for obtaining a ‘new' vial and having the first injection
administered in the allergist's office.
RELATED POLICY:
Emergency Assessment Policy (Location: Standing Delegated Orders section of Policy & Procedures)
(Leave blank if none)
(Leave blank if none)
ATTACHMENTS/ENCLOSURES:
Example of Protocol Orders (Texas ENT)
POLICY/PROCEDURE TRACKING FORM (to be added as last page of each P&P for documentation of
changes)
TITLE: ALLERGY SHOTS (IMMUNOTHERAPY)
Scope/Purpose: To provide guidelines to ensure safe administration of immunotherapy agents.
Division/Department: All Clinics
Policy/Procedure #:
Original Date: March 11, 2016
_X New _Replacement for:
Date Reviewed:
Date Revised:
CPIC Approved:
Board Approved:
Date of Revision
Description of Changes
Source: http://www.bvcaa.org/HSPP/Clinical%20P&P/Nursing/ALLERGY%20SHOTS%203_14_2016.pdf
vahabonline.com
EDUCATIONAL OBJECTIVE: Readers will manage psoriasis on the basis of its type and severity JENNIFER VILLASEÑOR-PARK, MD, PhD DAVID WHEELER, BS LISA GRANDINETTI, MD, FAAD University of Pittsburgh, Department of Dermatology, University of Pittsburgh School of Medicine, University of Pittsburgh, Department of Dermatology, Pittsburgh, PA Psoriasis:
headtotoehealthcare.org
Bleaching products fade areas of unwanted pigmentation by disrupting the production and distribution of melanin in the skin by targeting overactive melanocytes (pigment-producing cells). There are a variety of ways to accomplish this and studies have shown that the best results occur when using a combination of two or more of these ingredients. Tyrosinase Inhibitors: Tyrosinase is a copper enzyme which stimulates melanin production in the melanocyte. Most whitening agents fall under this category, interfering with the enzymes function and reducing pigment production in the melanocyte. Hydroquinone Hydroquinone is a hydroxyphenolic compound that has been widely used for skin lightening for 50 years. It is the only FDA approved product for bleaching and a prescription is required to obtain products with a concentration above 2%. It is the most widely studied and scientifically backed bleaching agent on the market, but its use can come at a cost. It can be very irritating to sensitive skin and cause pigmentation to darken and get worse. In very dark skin types, long term use of highly concentrated products (4% or more) can lead to a development of Exogenous Ochronosis, "an irreversible disfiguring cosmetic problem." It is also very difficult to stabilize and can oxidize quickly if exposed to light and air. If a product containing Hydroquinone has darkened from an off-white or a creamy, pale yellow to a gold or brown color, it will no longer be effective and should be discarded. When using Hydroquinone, it is imperative to stay out of the sun for the treatment to work. Always wear a full spectrum sunblock and a hat. Exposure to sun deteriorates the Hydroquinone, rendering it ineffective. Kojic Acid Kojic Acid, a byproduct of the fermentation of rice, was first discovered in Japan in 1907. Kojic acid is the most common bleaching agent next to Hydroquinone. Kojic Acid is a natural and more gentle on the skin alternative to using Hydroquinone. It penetrates the upper skin layers and inhibits the production of epidermal melanin. Kojic Acid does pose a risk of causing allergic or sensitizing reactions in a small number of people. Azelaic Acid A natural skin brightener found in wheat, rye, and barley. Azelaic acid is most effective in concentrations of 20% - an amount that makes is near comparable in activity to 4% Hydroquinone. In a study consisting of 329 melasma sufferers, half were treated with a 20% Azelaic Acid solution, and half were treated with a 4% Hydroquinone solution. 56% of patients treated with AZA had good to favorable results while 73% treated with HQ had a similar result. When combined with Tretinoin or Alpha Hydroxy Acids, the results were noticeably improved. Azelaic Acid, is also antibacterial and anti-inflammatory, so it is also effective in the treatment of rosacea and acne. Arbutin (also known as Alpha Arbutin, Bearberry Extract or Uva Ursi Extract) A botanical, naturally-occuring cousin to Hydroquinone, Arbutin was first discovered in the Uva Ursi plant. Compared to HQ, Arbutin has been shown to be significantly less cytotoxic to the melanocyte — making it a much safer, yet very effective alternative to Hydroquinone. In a clinical trial involving Japanese women with melasma, a 3% concentration of Arbutin produced good to excellent results in 71.4% of patients within a three month period. Licorice Extract One of the safest and most gentle bleaching agents around, Licorice Extract is a potent anti-inflammatory and antioxidant. The ingredients responsible for the skin whitening aspect of the plan are known as Glabradin and Liquiritin. Liquiritin (in a 20% concentration) has been shown to provide good to excellent results in 70-90% of patients with hyperpigmentation and melasma — with minimal side effects and only minor irritation. Mulberry Extract Mulberry Extract is derived from the root bark of the mulberry tree, Morus Alba L. Studies have confirmed it to effectively reduce tyrosinase activity at much lower concentrations than either Hydroquinone or Kojic Acid. N-Acetyl Glucosamine N-Acetyl Glucosamine is a more stable form of Glucosamine, an agent most widely known as an arthritis treatment. Studies have recently shown it to successfully reduce the amount of melanin in melanocytes by blocking tyrosinase conversion and its results improve significantly when combined with Niacinamide. Inhibition of Melanosome Transfer: These skin lightening agents lighten unwanted pigmentation by interfering with the transfer or melanosomes from the melanocyte to the keratinocytes. Niacinamide or Nicotinamide is a biologically active form of Niacin (Vitamin B3) that has been shown to interrupt melanocyte transfer by 35-68%.