Vahabonline.com
EDUCATIONAL OBJECTIVE: Readers will manage psoriasis on the basis of its type and severityJENNIFER VILLASEÑOR-PARK, MD, PhD DAVID WHEELER, BS LISA GRANDINETTI, MD, FAAD University of Pittsburgh, Department of Dermatology, University of Pittsburgh School of Medicine, University of Pittsburgh, Department of Dermatology, Pittsburgh, PA Psoriasis:
Evolving treatment for a complex disease
Much has changed in our understand-
ing of psoriasis over the past decade, The cutaneous manifestations of psoriasis can vary in which is having a major effect on its treatment. morphology and severity, and therapy should be tailored Although topical corticosteroids and photo- accordingly. Biologic agents are important new options therapy remain mainstays of treatment, a va- for treating patients with the most severe forms of the riety of biologic agents have given new hope disease. All physicians should be aware that severe psori- to those with the most severe forms of the asis may increase cardiovascular morbidity and the risk of disease. We are also beginning to understand death, and preventive strategies for patients with severe that patients with psoriasis are at greater risk disease should be considered. of cardiovascular disease, though the exact na- ture of that risk and how we should respond remains unclear. Finally, genome-wide associa- tion studies are just beginning to unravel the Studies in the past 10 years have uncovered a link be- genetic basis of psoriasis.
tween psoriasis, metabolic syndrome, and cardiovascular In this paper, we review the epidemiology disease. Interestingly, the risk grows less with age; patients and impact of psoriasis, current views of its at greatest risk are young men with severe psoriasis.
pathogenesis, its varied clinical forms, and its The most common presentation of psoriasis is plaque ■ Psoriasis imPosEs a grEat burdEn
psoriasis. However, there are several other clinical varia- tions of psoriasis, each of which has a distinct response Psoriasis is common, with a reported preva- to treatment and may be associated with significant sys- lence ranging from approximately 2%1 to temic symptoms.
4.7%.2 It can manifest at any age, but it is most common in two age groups, ie, 20 to 30 years Tumor necrosis factor inhibitors should be considered and 50 to 60 years.
first-line in the treatment of psoriatic arthritis.
For the patient, the burden is great, affect- ing physical, psychological, and occupational well-being. In fact, patients with psoriasis re- Phototherapy and systemic medications including metho- port quality-of-life impairment equal to or trexate, acitretin (Soriatane), cyclosporine (Gengraf, worse than that in patients with cancer or heart Neoral, Sandimmune), and biologic agents are the most disease.3,4 Notably, functional disability second- effective treatments for moderate-to-severe psoriasis.
ary to psoriatic arthritis has been reported in up to 19% of psoriatic arthritis patients, and this negatively affects quality of life.5 In 2004, the annual direct medical costs of psoriasis in the United States were estimated to exceed $1 billion. Its indirect costs, mea- sured as missed days and loss of productivity at CLEVELAND CLINIC JOURNAL OF MEDICINE VOLUME 79 • NUMBER 6 JUNE 2012 work, are estimated to exceed the direct costs ■ a vicious circLE of infLammation
by $15 billion annually.6,7 and kEratinocytE ProLifEration
Linked to cardiovascular and other diseases
The hallmark of plaque psoriasis is chronic Studies in the past 10 years have uncovered a inflammation in the skin, leading to keratino- link between psoriasis, metabolic syndrome, cyte proliferation. and cardiovascular disease.8–13 Specifically, External and internal triggers that have patients with severe psoriasis are at higher been identified include cutaneous injury (eg, risk of myocardial infarction and cardiovas- sunburn, drug rash, viral exanthems), infec- cular death than control patients. Interest- tions (eg, streptococcal), hypocalcemia, preg- ingly, the risk decreases with age; patients at nancy, psychogenic stress, drugs (eg, lithium, greatest risk are young men with severe pso- interferon, beta-blockers, and antimalarials), alcohol, smoking, and obesity.20–23 In a large cohort study in the United King- As reviewed by Nestle et al,24 the initiation dom7 comparing patients with and without of lesion formation is still poorly understood psoriasis, the hazard ratio for cardiovascular but is thought to occur when a trigger (physi- death in patients with severe psoriasis was 1.57 cal trauma, bacterial product, cellular stress) (95% confidence interval 1.26–1.96). This causes DNA to be released from keratinocytes. translated to 3.5 excess deaths per 1,000 pa- DNA forms a complex with the antimicro- tient-years. These patients were also at higher bial protein LL-37 and activates plasmacytoid risk of death from malignancies, chronic lower dendritic cells (PDCs) via toll-like receptor respiratory disease, diabetes, dementia, infec- 9. Activated PDCs release type I interferons, tion, kidney disease, and unknown causes.
which in turn activate myeloid dendritic cells. How much of the risk is due to psoriasis Myeloid dendritic cells release IL-20 locally, itself, its treatments, associated behaviors, or which speeds keratinocyte proliferation. other factors requires more study. However, A subset of myeloid dendritic cells leaves some evidence points to the dysregulation of the dermis and migrates to local lymph nodes, Primary care
the immune system, notably chronic eleva- where they release IL-23 and activate naive tion of pro-inflammatory cytokines.
T cells. T helper 1 (Th1) and Th17 cells are providers
Psoriasis and its comorbid conditions are recruited to the lesions and begin producing should consider thought to arise from chronically elevated lev- numerous cytokines, including interferon
screening
els of cytokines such as tumor necrosis factor gamma, IL-17, and IL-22. This cytokine mi- alpha (TNF-alpha), interleukin 1 beta (IL-1 lieu increases keratinocyte proliferation and patients with
beta), and IL-17. These cytokines impair insu- causes the keratinocytes to secrete antimicro- severe psoriasis lin signaling, deregulate lipid metabolism, and bial proteins (LL-37, beta defensins), chemo-
increase atherosclerotic changes in the coro- kines, and S100 proteins. These soluble fac- for metabolic
nary, cerebral, and peripheral arteries. In ad- tors have three main functions: stimulation of disorders and
dition, several other diseases that involve the dendritic cells to release more IL-23, recruit- cardiovascular immune system occur more frequently with ment of neutrophils to the epidermis, and ac-
psoriasis, including Crohn disease, ulcerative tivation of dermal fibroblasts. risk factors
colitis, lymphoma, obesity, and type 2 diabe- This cycle of keratinocytes activating den- dritic cells, dendritic cells activating T cells, In view of the prevalence of these comor- and T cells activating keratinocytes appears to bid conditions and the risks they pose, prima- be the main force maintaining the disease.24 It ry care physicians should consider screening is unclear, however, whether this applies to all patients with severe psoriasis for metabolic forms of psoriasis or only to plaque psoriasis.
disorders and cardiovascular risk factors and promptly begin preventive therapies.19 Un- genetic factors discovered
fortunately, to date, there are no consensus In recent years, genome-wide association guidelines as to the appropriate screening tests studies have identified at least 10 psoriasis-sus- or secondary cardiovascular preventive mea- ceptibility loci that involve functioning of the sures for patients with severe psoriasis.
immune system.25 These genes include those 414 CLEVELAND CLINIC JOURNAL OF MEDICINE VOLUME 79 • NUMBER 6 JUNE 2012
VILLASEÑOR-PARK AND COLLEAGUES
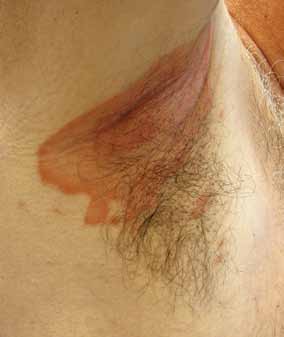
FIGURE 2. Patient with inverse psoriasis of
Photo courtesy of JosePh c. english iii, MD.
demarcated, scaly, pink-to-red plaques of vari-
ous sizes with a relatively symmetric distribu-
tion. Involvement of the extensor surfaces Psoriasis has
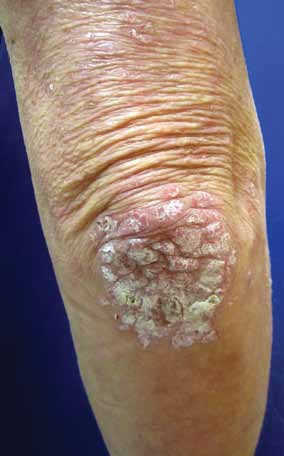
FIGURE 1. Well-demarcated erythematous,
scaly plaques characteristic of plaque pso-
such as the elbows and knees and of the scalp, several clinical
riasis on the elbow.
trunk, and intergluteal cleft is common.
variants,
Plaques can persist for several months to
years, even in the same location, and only each with a
of the major histocompatibility complex, cyto-
about 5% of patients report complete remis- distinct course
kines, receptors, and beta-defensins.
sion for up to 5 years.
Of specific interest, polymorphisms in the
and response
IL-12/IL-13 receptor, the p40 subunit of IL-
to treatment
12 and IL-23, and the p19 subunit of IL-23 Involvement of the skin folds, including the
strongly associate with psoriasis, supporting axillary, genital, perineal, intergluteal, and in-
their critical role in the disease process and framammary regions with pink-to-red plaques
providing targets for medical therapy.26
with minimal scale is the main clinical feature
of inverse psoriasis (FIGURE 2). Absence of sat-
■ Psoriasis Has sEvEraL
ellite pustules clinically distinguishes it from
Psoriasis has several clinical variants, each guttate psoriasis
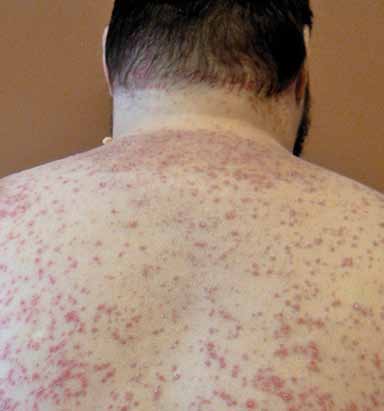
with a distinct clinical course and response to Guttate psoriasis (named for its droplet-
treatment.27 Moreover, many patients present shaped lesions) presents abruptly with 1-mm
with more than one variant.
to 10-mm pink papules with associated fine
scale over the trunk and extremities (FIGURE 3).
This variant occurs in fewer than 2% of pa-
Plaque psoriasis (FIGURE 1) accounts for more tients with psoriasis, who are usually younger
than 80% of cases. It is characterized by well-
than 30 years. It is often preceded 2 to 3 weeks
CLEVELAND CLINIC JOURNAL OF MEDICINE VOLUME 79 • NUMBER 6 JUNE 2012
earlier by an upper respiratory tract infection
with group A beta-hemolytic streptococci.
Approximately 1% to 2.25% of all patients
with psoriasis develop this severe form, affect-
ing more than 75% of the body surface area. It
presents as generalized erythema, which is the
most prominent feature, and it is often associ-
ated with superficial desquamation, hair loss,
nail dystrophy, and systemic symptoms such as
fever, chills, malaise, or high-output cardiac
failure. There may be a history of preceding
characteristic psoriatic plaques, recent with-
drawal of treatment (usually corticosteroids),
phototoxicity, or infection.
Conversely, approximately 25% of all pa-
tients with erythroderma have underlying
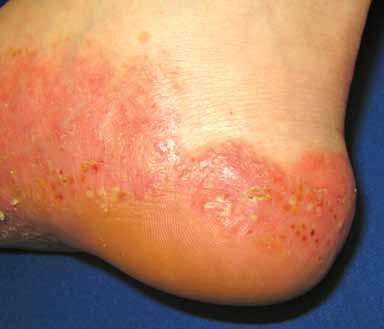
Pustular psoriasis (FIGURE 4) is uncommon. The
predominant lesions are large collections of
neutrophils in the stratum corneum that clini-
cally present as sterile pustules. The pustules
FIGURE 3. Guttate psoriasis with characteristic erythema-
may be localized within or at the edge of exist-
tous, scaly papules and small plaques on the back.
ing psoriatic plaques or may present as a gen-
Photo courtesy of laura K. ferris, MD, PhD.
eralized eruption.
There are several forms of pustular pso-
riasis, including generalized pustular psoriasis,
annular pustular psoriasis, impetigo herpeti-
formis (pustular psoriasis of pregnancy), and
palmoplantar pustulosis. However, there is
some evidence to suggest that palmoplantar
pustulosis may be distinct from psoriasis.29
Several triggers have been identified, in-
cluding pregnancy, rapid tapering of medica-
tions, hypocalcemia, infection, or topical ir-
Generalized pustular psoriasis, annular
pustular psoriasis, and impetigo herpetifor-
mis often present in association with fever
and other systemic symptoms and, if left un-
treated, can result in life-threatening com-
plications including bacterial superinfec-
tion, sepsis, dehydration, and, in rare cases,
acute respiratory distress secondary to aseptic
FIGURE 4. Erythematous plaques studded with pustules
Placental insufficiency resulting in still-
and red-brown macules on the acral surface of the foot in
birth or neonatal death and other fetal abnor-
malities can occur in severe pustular psoriasis
Photo courtesy of JosePh c. english iii, MD.
416 CLEVELAND CLINIC JOURNAL OF MEDICINE VOLUME 79 • NUMBER 6 JUNE 2012
VILLASEÑOR-PARK AND COLLEAGUES
Psoriatic arthritis is a seronegative inflam-
matory spondyloarthropathy that can result
in erosive arthritis in up to 57% of cases and
functional disability in up to 19%.32 Although
rare in the general population, it affects ap-
proximately 6% to 10% of psoriasis patients
and up to 40% of patients with severe psoria-
sis.33 In 70% of cases, psoriasis precedes the
onset of arthritis by about 10 years, and ap-
proximately 10% to 15% of patients simulta-
neously present with psoriasis and arthritis or
develop arthritis before skin involvement.5,34
Patients complain of joint discomfort that
is most prominent after periods of prolonged
rest. Patterns of involvement are extremely
variable but have been reported as an asym-
metric oligoarthritis (involving four or fewer
joints) or polyarthritis (involving more than
four joints) in most patients. A rheumatoid
arthritis-like presentation with a symmet-
ric polyarthropathy involving the small and
medium-sized joints has also been reported,
making it difficult to clinically distinguish this
from rheumatoid arthritis.
A distal interphalangeal-predominant pat-
tern is reported in 5% to 10% of patients. Ax-
ial disease resembling ankylosing spondylitis
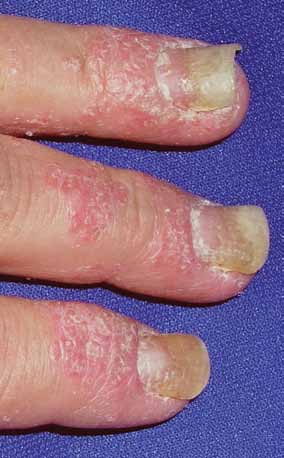
FIGURE 5. Nail pitting and onycholysis with
occurs only in 5% of patients. Arthritis muti-
surrounding psoriatic plaques along the
improvement
lans, characterized by severe, rapidly progres-
perionychium and proximal nail fold.
sive joint inflammation, joint destruction, and
in the PASI
Photo courtesy of JosePh c. english iii, MD.
deformity, occurs rarely. Enthesitis, ie, inflam-
mation at the point of attachment of tendons
is regarded as
or ligaments to bone, is present in up to 42%
ing more than 5% of the body surface or in-
volvement of the face, hands, feet, or genita- clinically
nail disease
The Psoriasis Area and Severity Index
Nail psoriasis occurs in 35% to 50% of patients
(PASI) is an objective measure used in clini-
and can be seen with all forms of psoriasis.1 In-
cal trials. It incorporates the amount of red-
volvement of the nail matrix can result in nail
ness, scaling, and induration of each psoriatic
pitting and leukonychia. Oil spots, subungual
lesion over the body surface involved. A 75%
hyperkeratosis, and distal onycholysis are the
improvement in the PASI score (PASI-75) is
result of disease involvement of the nail bed
regarded as clinically significant.37
(FIGURE 5). Up to 90% of patients with psoriatic
arthritis have nail changes, especially patients
■ Psoriasis is diagnosEd cLinicaLLy
with enthesitis.36
In most cases, the diagnosis of psoriasis is made
disease severity also varies
clinically and is straightforward. However, in
Disease severity also differs among patients. more difficult cases, biopsy may be needed. In
An estimated 80% of patients have mild to
moderate disease and 20% have moderate to
• The plaques of psoriasis may be confused
severe disease, which includes disease involv-
with squamous cell carcinoma in situ, der-
CLEVELAND CLINIC JOURNAL OF MEDICINE VOLUME 79 • NUMBER 6 JUNE 2012
matophyte infection, or cutaneous T-cell
lymphoma, especially if they are treat-
Steroid-sparing agents include vitamin D
analogues, retinoids, and tacrolimus ointment
• Guttate psoriasis may be difficult to distin-
guish from pityriasis rosea.
Vitamin D analogues and retinoids are
• Erythrodermic psoriasis must be distin-
thought to decrease keratinocyte proliferation
guished from other causes of erythroderma,
and enhance keratinocyte differentiation.39
including Sézary syndrome, pityriasis rubra
The vitamin D analogues are also considered
pilaris, and drug reactions.
first-line topical agents and include calcipot-
• Intertrigo, candidiasis, extramammary Pag-
riol (Dovonex), calcipotriene (Dovonex),
et disease, squamous cell carcinoma, and and calcitriol (Vectical). To prevent hypercal-
contact dermatitis all may mimic inverse
cemia, use of more than 100 g of vitamin D
analogues per week should be avoided.39
• Palmoplantar pustulosis may be difficult to
differentiate from dyshidrotic eczema.
treatment of inverse psoriasis
• Generalized pustular psoriasis should be and scalp psoriasis may be challenging
distinguished from a pustular drug eruption
The areas affected in inverse psoriasis, such
(acute generalized exanthematous pustular
as the genitalia and axillae, are more prone to
drug eruption or acute generalized exan-
side effects when potent topical steroids are
thematous pustulosis), impetigo, candidia-
used because of increased absorption and oc-
sis, or an autoimmune blistering disorder
clusion in these areas. Agents that minimize
such as pemphigus.
irritation and toxicity in sensitive areas, such
as topical tacrolimus, less-potent topical ste-
■ trEatmEnt of LimitEd disEasE
roids, or calcitriol, can be used.39
For scalp psoriasis, alternative vehicles
such as shampoos, gels, solutions, oils, sprays,
A topical corticosteroid, either by itself or and foams have improved patient compliance
Steroid-sparing combined with a steroid-sparing agent, is the and efficacy of treatment.40
agents include first-line therapy for patients with limited
vitamin D
disease. The potency required for treatment
■ PHototHEraPy for sEvErE disEasE
should be based on the extent of disease and
analogues,
on the location, the choice of vehicle, and the
narrow-band ultraviolet b
patient's preference and age.
Narrow-band ultraviolet B, ie, light confined
Several double-blind studies have assessed
to wavelengths of 311 to 313 nm, is a first-
retinoids, and
the efficacy of various topical corticosteroids line treatment for moderate to severe pso-
tacrolimus
in treating psoriasis. In general, super-potent riasis, either as monotherapy or in combina-
(class I) and potent (class II) topical corticoste-
tion with other treatments. It is an especially
roids are more efficacious than mild or moder-
attractive option in patients who are on
ate corticosteroids.38 Class I and class II steroids
medications or who have comorbidities that
include agents such as clobetasol propionate may preclude treatment with other systemic
0.05% (Temovate), betamethasone dipropio-
nate 0.05% (Diprolene), fluocinonide 0.05% The mechanism of action may be via im-
(Lidex), and desoximetasone 0.25% (Topicort).
munosuppressive effects on Langerhans cells,
Use of class I steroids should be limited alteration of cytokines and adhesion mol-
to an initial treatment course of twice-daily
ecules that lead to an increase in Th2 cells,
application for 2 to 4 weeks in an effort to
and induction of apoptosis of T lymphocytes.
avoid some of the local toxicities such as skin
Additionally, ultraviolet light affects the
atrophy, telangiectasia, and striae. Decreas-
proliferation and differentiation of keratino-
ing class I topical steroid use to 1 to 2 times
per week with the gradual introduction of a
Dosing is based on skin type, and treat-
steroid-sparing agent following the initial 2 to
ment usually involves two or three visits per
4 weeks of treatment is advised.
week for a total of 15 to 20 treatments, with
418 CLEVELAND CLINIC JOURNAL OF MEDICINE VOLUME 79 • NUMBER 6 JUNE 2012
VILLASEÑOR-PARK AND COLLEAGUES
additional therapy for maintenance. Adding
acitretin (Soriatane), with close monitoring In 1972, the US Food and Drug Administra-
of aspartate aminotransferase and alanine tion (FDA) approved methotrexate for treat-
aminotransferase levels and the patient's lipid
ing severe psoriasis.42 In studies of methotrex-
panel, can be considered in treatment-resis-
ate at doses of 15 to 20 mg weekly, 36% to
68% of patients with severe plaque psoriasis
achieved a PASI-75 score.40,42,47
Psoralen combined with ultraviolet a
Dosages of methotrexate for treating se-
Psoralen combined with ultraviolet A is an-
vere psoriasis range from 7.5 to 25 mg once
other option. It can be considered if narrow-
a week. Patients should also receive a folate
band ultraviolet B treatment fails. It is also supplement of 1 to 5 mg every day except the
useful for dark-skinned patients and those day they take methotrexate. The folate is to
with thicker plaques because ultraviolet A protect against gastrointestinal side effects,
penetrates deeper than ultraviolet B. Oral or
bone marrow suppression, and hepatic toxic-
topical treatment with psoralen is followed by
ity associated with methotrexate.
ultraviolet A treatment.
Other side effects of methotrexate include
The duration of remission is much longer
pulmonary fibrosis and stomatitis. Pregnancy,
with psoralen plus ultraviolet A than with nursing, alcoholism, chronic liver disease, im-
narrow-band ultraviolet B. However, this munodeficiency syndromes, bone-marrow hy-
treatment caries a significant risk of cutane-
poplasia, leukopenia, thrombocytopenia, ane-
ous squamous cell carcinoma and melanoma,
mia, and hypersensitivity to methotrexate are
especially in light-skinned people and those
all contraindications to methotrexate use.
who receive high doses of ultraviolet A (200
The National Psoriasis Foundation, in its
or more treatments) or cyclosporine.40,41,43–46
2009 guidelines for the use of methotrexate
Long-term effects include photoaging, lentigi-
in treating psoriasis,48 recommends obtaining
nes, and telangiectasias. As a consequence of
a complete blood cell count with platelets,
these well-established side effects, this treat-
blood urea nitrogen, creatinine, and liver
ment is used less frequently.
function tests at baseline and at 1- to 3-month All retinoids,
intervals thereafter.
including
cautions with phototherapy
Liver biopsies were previously recom-
Careful screening and caution should be used
mended for patients receiving methotrexate acitretin, are
in patients who have:
long-term when the cumulative dose of thera- in pregnancy
• Fair skin that tends to burn easily
py reached 1.5 g. However, given the invasive category X
• A history of arsenic intake or treatment nature of the liver biopsy procedure and the
with ionizing radiation
low incidence of methotrexate-induced hepa- and should
• A history of use of photosensitizing medi-
totoxicity, this recommendation has been re- therefore
cations (fluoroquinolone antibiotics, doxy-
For patients with no significant risk fac- be avoided
• A history of melanoma or atypical nevi
tors for hepatic toxicity (eg, obesity, diabetes, during
• Multiple risk factors for melanoma
hyperlipidemia, hepatitis, or history of or cur-
• A history of nonmelanoma skin cancer
rent alcohol consumption) and normal liver pregnancy
• Immunosuppression due to organ trans-
function tests, liver biopsy should be consid-
ered when a cumulative methotrexate dose of
3.5 to 4.0 g is reached. Alternatively, one may
■ oraL tHEraPiEs for sEvErE Psoriasis
choose to continue to monitor the patient
without liver biopsy or to switch to another
Patients who have severe psoriasis—ie, af-
medication, if possible.42,48
fecting more than 5% of the body surface or
Patients at high risk should be monitored
debilitating disease affecting the palms, soles,
more carefully, and liver biopsy should be con-
or genitalia—are best managed with systemic
sidered soon after starting methotrexate and
medications, especially if they do not have ac-
repeated after every 1.0 to 1.5 g.48
cess to phototherapy.20
No reliable noninvasive measures to eval-
CLEVELAND CLINIC JOURNAL OF MEDICINE VOLUME 79 • NUMBER 6 JUNE 2012
uate for liver fibrosis are routinely available tive as a maintenance therapy, usually after
in the United States. Serial measurements of
the disease has been stabilized by agents such
serum type III procollagen aminopeptide have
as cyclosporine, or in combination with other
been reported to correlate with the risk of treatments such as phototherapy.42 Acitretin
developing liver fibrosis; however, this test is
has been shown to be effective in patients
readily available only in Europe.49
with pustular psoriasis.54
Acitretin does not alter the immune sys-
tem and has not been shown to have signifi-
Cyclosporine (Gengraf, Neoral, Sandimmune)
cant cumulative toxicities. Serum triglycer-
is very effective for treating psoriasis, espe-
ides are monitored closely, since acitretin can
cially erythrodermic psoriasis. It is often used
lead to hypertriglyceridemia.
only short-term or as a bridge to other main-
All retinoids, including acitretin, are in
tenance therapies because it has a rapid onset
pregnancy category X and should therefore be
and because long-term therapy (3 to 5 years) is
avoided during pregnancy. Although its half-
associated with a risk of glomerulosclerosis.50
life is only 49 hours, acitretin may be trans-
Cyclosporine works by decreasing T-cell formed to etretinate either spontaneously or
activation by binding cyclophilin, which leads
as a result of alcohol ingestion. Etretinate has
to inhibition of transcription of calcineu-
a half-life of 168 days and can take up to 3
rin and nuclear factor of activated T cells.51
years to be eliminated from the body. There-
Given at doses of 2.5 to 5 mg/kg/day, cyclo-
fore, acitretin is contraindicated in women
sporine has been shown to result in rapid im-
who plan to become pregnant or who do not
provement in up to 80% to 90% of psoriatic
agree to use adequate contraception for 3 years
after the drug is discontinued.42
The initial recommended dose of cyclo-
sporine is usually 2.5 to 3 mg/kg/day in two
divided doses, which is maintained for 4 weeks
Advances in our understanding of the patho-
and then increased by 0.5 mg/kg/day until the
genesis of psoriasis have resulted in more spe-
In cases of
disease is stable.42
cific, targeted therapy.
Nephrotoxicity and hypertension are cyclo-
Alefacept (Amevive) is a human Fc IgG1
psoriatic
sporine's most serious side effects. Blood receptor fused to the alpha subunit of LFA3. It
arthritis,
urea nitrogen, creatinine, and blood pressure
binds to CD2, blocks costimulatory signaling,
evaluation by a should be monitored at baseline and then and induces apoptosis in activated memory T
twice a month for the first 3 months and once
rheumatologist monthly thereafter. Liver function tests, com- Alefacept was the first biologic agent ap-
is highly
plete blood cell count, lipid profile, magne-
proved by the FDA for the treatment of pso-
sium, uric acid, and potassium should also be
riasis and one of the few biologic agents to in-
recommended checked every month.
duce long-term remission.55 However, its use
Cyclosporine also increases the risk of cu-
has declined because few patients achieved
taneous squamous cell carcinoma, especially significant clearance of their psoriasis and its
in patients who have received psoralen plus
onset of action was much slower than that of
ultraviolet A treatment.42
other medications.56
Patients with hypersensitivity to cyclospo-
The currently approved biologic therapies
rine, a history of chronic infection (eg, tuber-
commonly used for moderate to severe pso-
culosis, hepatitis B, hepatitis C), renal insuf-
riasis include the TNF-alpha inhibitors and
ficiency, or a history of systemic malignancy
should not receive cyclosporine.
The TNF-alpha inhibitors include inflix-
imab (Remicade), etanercept (Enbrel), and
adalimumab (Humira). They are generally
Acitretin, an oral retinoid, has been used for
well tolerated and highly effective. Howev-
several years to treat psoriasis. Its onset is slow,
er, TNF-alpha inhibitors and other biologic
typically ranging from 3 to 6 months, and its
agents are contraindicated in patients with se-
effects are dose-dependent. It is most effec-
rious infection, a personal history or a family
420 CLEVELAND CLINIC JOURNAL OF MEDICINE VOLUME 79 • NUMBER 6 JUNE 2012
VILLASEÑOR-PARK AND COLLEAGUES
history in a first-degree relative of demyelinat-
IL-12 and IL-23, which have been shown to
ing disease, or class III or IV congestive heart
be at increased levels in psoriatic lesions and
failure. Patients should be screened for active
important for the pathogenesis of psoriasis.
infection, including tuberculosis and hepatitis
Between 66% and 76% of patients treated
B, since reactivation has been reported fol-
with ustekinumab achieved significant clear-
lowing initiation of TNF-alpha inhibitors.1
ance of their disease after 12 weeks of treat-
Adalimumab is a human monoclonal an-
ment in two large phase III multicenter, ran-
tibody against TNF-alpha. It binds to soluble
domized, double-blind, placebo-controlled
and membrane-bound TNF-alpha and pre-
vents it from binding to p55 and p75 cell-sur-
Dosing of ustekinumab is weight-based. For
face TNF receptors.
those weighing less than 100 kg, ustekinumab
The dosing schedule for adalimumab is 80
is given at 45 mg subcutaneously at baseline,
mg subcutaneously for the first week, followed
at 4 weeks, and every 12 weeks thereafter. The
by 40 mg subcutaneously the next week, and
same dosing schedule is used for those weigh-
then 40 mg subcutaneously every 2 weeks ing more than 100 kg, but the dose is increased
Etanercept is a recombinant human TNF-
Guidelines for monitoring patients while
alpha receptor (p75) protein fused with the Fc
on ustekinumab are similar to those for other
portion of IgG1, which binds to soluble TNF-
biologic agents. Information on long-term
alpha.57 Dosing for etanercept is 50 mg subcu-
toxicities is still being collected. However,
taneously twice weekly for the first 12 weeks,
injection-site reactions, serious infections,
followed by 50 mg weekly thereafter.
malignancies, and a single case of reversible
Infliximab is a chimeric antibody com-
posterior leukoencephalopathy have been re-
posed of a human IgG1 constant region fused
to a mouse variable region that binds to both
While biologic agents are significantly
soluble and membrane-bound TNF-alpha.58
more expensive than the conventional thera-
Infliximab is given as an infusion at a dose of
pies discussed above and insurance coverage
5 mg/kg over 2 to 3 hours at weeks 0, 2, and 6,
for these agents varies, they have demonstrat- Before
and then every 8 weeks thereafter.
ed superior efficacy and may be indicated for starting a
Efficacy of TNF inhibitors. There are patients with recalcitrant moderate to severe
no specific guidelines for the sequence of ini-
psoriasis for whom multiple types of treatment TNF-alpha
tiation of TNF inhibitors because no studies
have failed.
inhibitor,
have directly compared the efficacy of these
screen for
medications. However, response to infliximab
■ for Psoriatic artHritis:
is relatively rapid compared with adalimumab
infection,
In a phase III clinical trial,59 as many as
For patients with known or questionable pso-
80% of patients achieved PASI-75 clear-
riatic arthritis, evaluation by a rheumatologist including
ance of their psoriasis after three doses of in-
is highly recommended.
fliximab. Interestingly, only 61% of patients
Nonsteroidal anti-inflammatory drugs
maintained PASI-75 clearance by week 50. (NSAIDs) are usually first-line in the treat- and hepatitis B
This loss of efficacy of infliximab is also re-
ment of mild psoriatic arthritis. If after 2 to
ported with other TNF-alpha inhibitors and is
3 months of therapy with NSAIDs no benefit
thought to be secondary to the development
is achieved, treatment with methotrexate as
of antibodies to the drugs. For infliximab, this
monotherapy is a practical consideration be-
loss of efficacy is less when infliximab is given
cause of its low cost. However, methotrexate
continuously rather than on an as-needed ba-
as a monotherapy has not been shown to pre-
sis. Simultaneous treatment with methotrex-
vent radiologic progression of disease.5,32
ate is also thought to decrease the develop-
The TNF-alpha inhibitors have been
ment of antibodies to infliximab.60
shown to have similar efficacy when compared
Ustekinumab is an monoclonal antibody
among each other in the treatment of psoriat-
directed against the common p40 subunit of
ic arthritis.32,63 Based on radiologic evidence,
CLEVELAND CLINIC JOURNAL OF MEDICINE VOLUME 79 • NUMBER 6 JUNE 2012
ustekinumab has not shown to be as effica-
because of their associated systemic symp-
cious as the TNF-alpha inhibitors for treating
toms. Care should be taken to rule out sepsis,
psoriatic arthritis. Therefore, TNF inhibitors
as this is a reported trigger of erythrodermic
should be considered first-line in the treat-
ment of psoriatic arthritis.21,64
Systemic medications with a quick onset,
Few studies have been done on the efficacy
such as oral cyclosporine, are recommended.
or sequence of therapies that should be used
Infliximab has also been reported to be benefi-
in the treatment of psoriatic arthritis. The cial because of its rapid onset.28
American Academy of Dermatology's Pso-
riasis Guidelines of Care recommend adding
■ trEatmEnt basEd on tHE tyPE
a TNF-alpha inhibitor or switching to a TNF-
and tHE sEvErity of Psoriasis
alpha inhibitor if no significant improvement
is achieved after 12 to 16 weeks of treatment
The treatment of psoriasis can be as complex
with oral methotrexate.20
as the disease it itself and should be based on
the type and the severity of psoriasis. Recogni-
■ for ErytHrodErmic Psoriasis:
tion of the various manifestations of psoriasis
mEdications tHat act PromPtLy
is important for effective treatment. However,
in patients with moderate to severe psoriasis,
The care of erythrodermic psoriatic patients
atypical presentations, or recalcitrant disease,
is distinct from that of other psoriatic patients
referral to a specialist is recommended.
13. Tobin AM, Veale DJ, Fitzgerald O, et al. Cardiovascular disease and
risk factors in patients with psoriasis and psoriatic arthritis. J Rheu-
1. Menter A, Gottlieb A, Feldman SR, et al. Guidelines of care for
matol 2010; 37:1386–1394.
the management of psoriasis and psoriatic arthritis: section 1.
14. Najarian DJ, Gottlieb AB. Connections between psoriasis and
Overview of psoriasis and guidelines of care for the treatment of
Crohn's disease. J Am Acad Dermatol 2003; 48:805–821.
psoriasis with biologics. J Am Acad Dermatol 2008; 58:826–850.
15. Neimann AL, Shin DB, Wang X, Margolis DJ, Troxel AB, Gelfand
2. Christophers E. Psoriasis—epidemiology and clinical spectrum. Clin
JM. Prevalence of cardiovascular risk factors in patients with pso-
Exp Dermatol 2001; 26:314–320.
riasis. J Am Acad Dermatol 2006; 55:829–835.
3. Rapp SR, Feldman SR, Exum ML, Fleischer AB Jr, Reboussin DM.
16. Shapiro J, Cohen AD, Weitzman D, Tal R, David M. Psoriasis and
Psoriasis causes as much disability as other major medical diseases.
cardiovascular risk factors: a case-control study on inpatients com-
J Am Acad Dermatol 1999; 41:401–407.
paring psoriasis to dermatitis. J Am Acad Dermatol 2012; 66:252–
4. Weiss SC, Kimball AB, Liewehr DJ, Blauvelt A, Turner ML, Emanuel
EJ. Quantifying the harmful effect of psoriasis on health-related
17. Gelfand JM, Shin DB, Neimann AL, Wang X, Margolis DJ, Troxel
quality of life. J Am Acad Dermatol 2002; 47:512–518.
AB. The risk of lymphoma in patients with psoriasis. J Invest Der-
5. Garg A, Gladman D. Recognizing psoriatic arthritis in the derma-
matol 2006; 126:2194–2201.
tology clinic. J Am Acad Dermatol 2010; 63:733–748.
18. Chen YJ, Wu CY, Chen TJ, et al. The risk of cancer in patients with
6. Kimball AB, Yu AP, Signorovitch J, et al. The effects of adalimumab
psoriasis: a population-based cohort study in Taiwan. J Am Acad
treatment and psoriasis severity on self-reported work productivity
Dermatol 2011; 65:84–91.
and activity impairment for patients with moderate to severe pso-
19. Friedewald VE, Cather JC, Gelfand JM, et al. AJC editor's con-
riasis. J Am Acad Dermatol 2012; 66:e67–e76.
sensus: psoriasis and coronary artery disease. Am J Cardiol 2008;
7. Schmitt JM, Ford DE. Work limitations and productivity loss are
associated with health-related quality of life but not with clinical
20. American Academy of Dermatology Work Group; Menter A, Kor-
severity in patients with psoriasis. Dermatology 2006; 213:102–110.
man NJ, Elmets CA, et al. Guidelines of care for the management
8. Gelfand JM, Neimann AL, Shin DB, Wang X, Margolis DJ, Troxel
of psoriasis and psoriatic arthritis: section 6. Guidelines of care for
AB. Risk of myocardial infarction in patients with psoriasis. JAMA
the treatment of psoriasis and psoriatic arthritis: case-based pre-
sentations and evidence-based conclusions. J Am Acad Dermatol
9. Abuabara K, Azfar RS, Shin DB, Neimann AL, Troxel AB, Gelfand
2011; 65:137–174.
JM. Cause-specific mortality in patients with severe psoriasis: a
21. Mallbris L, Larsson P, Bergqvist S, Vingård E, Granath F, Ståhle M.
population-based cohort study in the U.K. Br J Dermatol 2010;
Psoriasis phenotype at disease onset: clinical characterization of
400 adult cases. J Invest Dermatol 2005; 124:499–504.
10. Ahlehoff O, Gislason GH, Charlot M, et al. Psoriasis is associated
22. Armstrong AW, Armstrong EJ, Fuller EN, Sockolov ME, Voyles
with clinically significant cardiovascular risk: a Danish nationwide
SV. Smoking and pathogenesis of psoriasis: a review of oxida-
cohort study. J Intern Med 2011; 270:147–157.
tive, inflammatory and genetic mechanisms. Br J Dermatol 2011;
11. Lin HW, Wang KH, Lin HC, Lin HC. Increased risk of acute myocar-
dial infarction in patients with psoriasis: a 5-year population-based
23. Qureshi AA, Dominguez PL, Choi HK, Han J, Curhan G. Alcohol
study in Taiwan. J Am Acad Dermatol 2011; 64:495–501.
intake and risk of incident psoriasis in US women: a prospective
12. Bremmer S, Van Voorhees AS, Hsu S, et al; National Psoriasis
study. Arch Dermatol 2010; 146:1364–1369.
Foundation. Obesity and psoriasis: from the Medical Board of the
24. Nestle FO, Kaplan DH, Barker J. Psoriasis. N Engl J Med
National Psoriasis Foundation. J Am Acad Dermatol 2010; 63:1058–
25. Genetic Analysis of Psoriasis Consortium & the Wellcome Trust
422 CLEVELAND CLINIC JOURNAL OF MEDICINE VOLUME 79 • NUMBER 6 JUNE 2012
VILLASEÑOR-PARK AND COLLEAGUES
Case Control Consortium 2; Strange A, Capon F, Spencer CC, et al. A
47. Flytström I, Stenberg B, Svensson A, Bergbrant IM. Methotrexate
genome-wide association study identifies new psoriasis susceptibil-
vs. ciclosporin in psoriasis: effectiveness, quality of life and safety. A
ity loci and an interaction between HLA-C and ERAP1. Nat Genet
randomized controlled trial. Br J Dermatol 2008; 158:116–121.
2010; 42:985–990.
48. Kalb RE, Strober B, Weinstein G, Lebwohl M. Methotrexate and
26. Nair RP, Duffin KC, Helms C, et al; Collaborative Association Study
psoriasis: 2009 National Psoriasis Foundation Consensus Conference.
of Psoriasis. Genome-wide scan reveals association of psoriasis with
J Am Acad Dermatol 2009; 60:824–837.
IL-23 and NF-kappaB pathways. Nat Genet 2009; 41:199–204.
49. Zachariae H, Heickendorff L, Søgaard H. The value of amino-
27. Griffiths CE, Christophers E, Barker JN, et al. A classification of
terminal propeptide of type III procollagen in routine screening for
psoriasis vulgaris according to phenotype. Br J Dermatol 2007;
methotrexate-induced liver fibrosis: a 10-year follow-up. Br J Der-
matol 2001; 144:100–103.
28. Rosenbach M, Hsu S, Korman NJ, et al; National Psoriasis Founda-
50. Lowe NJ, Wieder JM, Rosenbach A, et al. Long-term low-dose cyclo-
tion Medical Board. Treatment of erythrodermic psoriasis: from
sporine therapy for severe psoriasis: effects on renal function and
the medical board of the National Psoriasis Foundation. J Am Acad
structure. J Am Acad Dermatol 1996; 35:710–719.
Dermatol 2010; 62:655–662.
51. Gottlieb AB, Grossman RM, Khandke L, et al. Studies of the effect
29. Mrowietz U, van de Kerkhof PC. Management of palmoplantar pus-
of cyclosporine in psoriasis in vivo: combined effects on activated T
tulosis: do we need to change? Br J Dermatol 2011; 164:942–946.
lymphocytes and epidermal regenerative maturation. J Invest Der-
30. Kluger N, Bessis D, Guillot B, Girard C. Acute respiratory distress
matol 1992; 98:302–309.
syndrome complicating generalized pustular psoriasis (psoriasis-as-
52. Ellis CN, Fradin MS, Messana JM, et al. Cyclosporine for plaque-type
sociated aseptic pneumonitis). J Am Acad Dermatol 2011; 64:1154–
psoriasis. Results of a multidose, double-blind trial. N Engl J Med
31. Roth MM. Pregnancy dermatoses: diagnosis, management, and
53. Faerber L, Braeutigam M, Weidinger G, et al. Cyclosporine in severe
controversies. Am J Clin Dermatol 2011; 12:25–41.
psoriasis. Results of a meta-analysis in 579 patients. Am J Clin Der-
32. Gottlieb A, Korman NJ, Gordon KB, et al. Guidelines of care for the
matol 2001; 2:41–47.
management of psoriasis and psoriatic arthritis: section 2. Psoriatic
54. Ozawa A, Ohkido M, Haruki Y, et al. Treatments of generalized
arthritis: overview and guidelines of care for treatment with an
pustular psoriasis: a multicenter study in Japan. J Dermatol 1999;
emphasis on the biologics. J Am Acad Dermatol 2008; 58:851–864.
33. Ogdie A, Gelfand JM. Identification of risk factors for psoriatic
55. Krueger GG, Ellis CN. Alefacept therapy produces remission for
arthritis: scientific opportunity meets clinical need. Arch Dermatol
patients with chronic plaque psoriasis. Br J Dermatol 2003; 148:
34. Gelfand JM, Gladman DD, Mease PJ, et al. Epidemiology of pso-
56. Lebwohl M, Christophers E, Langley R, Ortonne JP, Roberts J,
riatic arthritis in the population of the United States. J Am Acad
Griffiths CE; Alefacept Clinical Study Group. An international,
Dermatol 2005; 53:573.
randomized, double-blind, placebo-controlled phase 3 trial of intra-
35. Moll JM, Wright V. Psoriatic arthritis. Semin Arthritis Rheum 1973;
muscular alefacept in patients with chronic plaque psoriasis. Arch
Dermatol 2003; 139:719–727.
36. McGonagle D. Enthesitis: an autoinflammatory lesion linking nail
57. Gottlieb AB, Matheson RT, Lowe N, et al. A randomized trial of
and joint involvement in psoriatic disease. J Eur Acad Dermatol
etanercept as monotherapy for psoriasis. Arch Dermatol 2003;
Venereol 2009; 23(suppl 1):9–13.
37. Feldman SR, Krueger GG. Psoriasis assessment tools in clinical trials.
58. Gottlieb AB, Masud S, Ramamurthi R, et al. Pharmacodynamic
Ann Rheum Dis 2005; 64(suppl 2):ii65–ii68.
and pharmacokinetic response to anti-tumor necrosis factor-alpha
38. Mason J, Mason AR, Cork MJ. Topical preparations for the treat-
monoclonal antibody (infliximab) treatment of moderate to severe
ment of psoriasis: a systematic review. Br J Dermatol 2002; 146:351–
psoriasis vulgaris. J Am Acad Dermatol 2003; 48:68–75.
59. Reich K, Nestle FO, Papp K, et al; EXPRESS study investigators. Inf-
39. Menter A, Korman NJ, Elmets CA, et al; American Academy of Der-
liximab induction and maintenance therapy for moderate-to-severe
matology. Guidelines of care for the management of psoriasis and
psoriasis: a phase III, multicentre, double-blind trial. Lancet 2005;
psoriatic arthritis. Section 3. Guidelines of care for the management
and treatment of psoriasis with topical therapies. J Am Acad Der-
60. Menter A, Feldman SR, Weinstein GD, et al. A randomized compari-
son of continuous vs. intermittent infliximab maintenance regimens
matol 2009; 60:643–659.
over 1 year in the treatment of moderate-to-severe plaque psoria-
40. Zivkovich AH, Feldman SR. Are ointments better than other ve-
sis. J Am Acad Dermatol 2007; 56:31.e1–31.e15.
hicles for corticosteroid treatment of psoriasis? J Drugs Dermatol
61. Papp KA, Langley RG, Lebwohl M, et al; PHOENIX 2 study investiga-
2009; 8:570–572.
tors. Efficacy and safety of ustekinumab, a human interleukin-12/23
41. Menter A, Korman NJ, Elmets CA, et al. Guidelines of care for the
monoclonal antibody, in patients with psoriasis: 52-week results
management of psoriasis and psoriatic arthritis: section 5. Guide-
from a randomised, double-blind, placebo-controlled trial (PHOE-
lines of care for the treatment of psoriasis with phototherapy and
NIX 2). Lancet 2008; 371:1675–1684.
photochemotherapy. J Am Acad Dermatol 2010; 62:114–135.
62. Leonardi CL, Kimball AB, Papp KA, et al; PHOENIX 1 study inves-
42. Menter A, Korman NJ, Elmets CA, et al. Guidelines of care for the
tigators. Efficacy and safety of ustekinumab, a human interleu-
management of psoriasis and psoriatic arthritis: section 4. Guide-
kin-12/23 monoclonal antibody, in patients with psoriasis: 76-week
lines of care for the management and treatment of psoriasis with
results from a randomised, double-blind, placebo-controlled trial
traditional systemic agents. J Am Acad Dermatol 2009; 61:451–485.
(PHOENIX 1). Lancet 2008; 371:1665–1674.
43. Murase JE, Lee EE, Koo J. Effect of ethnicity on the risk of develop-
63. Griffiths CE, Strober BE, van de Kerkhof P, et al; ACCEPT Study
ing nonmelanoma skin cancer following long-term PUVA therapy.
Group. Comparison of ustekinumab and etanercept for moderate-
Int J Dermatol 2005; 44:1016–1021.
to-severe psoriasis. N Engl J Med 2010; 362:118–128.
44. Stern RS, Lunder EJ. Risk of squamous cell carcinoma and methox-
64. Gottlieb A, Menter A, Mendelsohn A, et al. Ustekinumab, a human
salen (psoralen) and UV-A radiation (PUVA). A meta-analysis. Arch
interleukin 12/23 monoclonal antibody, for psoriatic arthritis: ran-
Dermatol 1998; 134:1582–1585.
domised, double-blind, placebo-controlled, crossover trial. Lancet.
45. Stern RS, Väkevä LH. Noncutaneous malignant tumors in the PUVA
follow-up study: 1975–1996. J Invest Dermatol 1997; 108:897–900.
46. Patel RV, Clark LN, Lebwohl M, Weinberg JM. Treatments for psoria-
ADDRESS: Lisa M. Grandinetti, MD, FAAD, Department of Dermatology,
sis and the risk of malignancy. J Am Acad Dermatol 2009; 60:1001–
University of Pittsburgh, Presby South Tower Suite 3880, 200 Lothrop
Street, Pittsburgh, PA 15213; e-mail [email protected].
CLEVELAND CLINIC JOURNAL OF MEDICINE VOLUME 79 • NUMBER 6 JUNE 2012
Source: http://vahabonline.com/wp-content/uploads/2014/10/sxdfgu____aws8er7t4y2th000.pdf
ssns.ca
Anna was visiting a friend in another city when strange things began to happen. She saw things that looked real, but later didn't seem real at all. She was so troubled by what she described as not feeling like herself that she woke her friend; together, they called Anna's parents. "She started to cry," says Anna's dad. "She was talking about things, but we didn't know where they were coming from."
mse.lab.scu.edu.cn
NIH Public AccessAuthor ManuscriptNat Rev Genet. Author manuscript; available in PMC 2010 November 1. NIH-PA Author Manuscript Published in final edited form as: Nat Rev Genet. 2010 May ; 11(5): 367–379. doi:10.1038/nrg2775. Synthetic Biology: Applications Come of Age Ahmad S. Khalil1 and James J. Collins1,2,*1 Howard Hughes Medical Institute, Department of Biomedical Engineering, Center forBioDynamics, and Center for Advanced Biotechnology, Boston University, Boston, MA 02215, USA