Biotranszformáció drug metabolism

Metabolism of Xenobiotics
Biotransformation is a process in which a foreign xenobiotic or an endogenious hormon, neuro-
transmitter, paracrine hormon is transformed to become more hydrophylic, consequently exretable.
Sometimes molecules are converted to active, more often to inactive formes.
It does not yield energy, but uses products of common metabolic reactions:
UDP-glucose, NADPH, SAM, PAPS, glutathione, acetyl-CoA, glycine, taurine
English name is drug metabolism or metabolism of xenobiotics, althouh there are endogenious
substrates, too.
It is ancient mechanism (3.5 billion years) found in bacteria, fungi, plants, animals
to get rid of foreign, harmful or endogenious regulatory molecules
preparation
conjugation
exretion
out of the organells, out of the cells
a.) secreted to bile then
glutathione conj.
not reabsorbed from gut
free OH, SH, NH2, COOH
b.) they are filtered in kidney
glycine, taurin, Gln conj.
and not reabsorbed from urine
Cytochrome P450 enzyme system
main family: at least 40% amino acid sequence homology
CYP1A1 subfamily: at least 55% amino acid sequence homology numbering of CYP and sources of enzymes: 1 -
human, higher animals
lower eucaryotic animals and fungi
11 gene family CYP proteins are found in humans and mammals All CYP enzymes together can catalize 60 different kind of reactions. In human and mammals CYP enzymes are membrane bound in ER and
1-4 gene family members have unimaginably broad and overlapping substrate specificity:
several enzyme can transform the same substrate, possibly by different way
one enzyme accepts huge amount of substrates
these enzymes transform plant poisons, medicines, chemicals, polutants
5-51 gene family members are specific, they transform endogenious compounds
Main localization of CYP enzymes:
liver, kidney, lung, intestine, skin
Genetic polimorphism often occurs: small alterations in amino acid sequence can affect
enzyme activity, stability, substrate specificity, affinity for substrates
leading to differences in medicine's tolerance
Liver failure results toxicity of usual doses of drugs.
Regulation of CYP enzymes is done by regulation of amount of enzymes:
induction-repression, mRNA stability, protein stability. Regulators are chemicals,
The same molecule: originating from food or spicy plant, hormon,
pesticides and herbicides, soil pollutants, water pollutants,
air pollutants and smoke, cigarette smoke,
medicines and food preservatives, colouring compounds, auxiliary materials
can act as substrate and inducer or repressor as well.
It causes drug side effects/nonwanted effects, the usual dose can become toxic

Cytochrome P450 enzyme system is a monooxygenase: 1 atom of oxygen is built in to the molecule or called mixed function oxygenase, because the other atom of oxygen forms water,
Cytochrome P450 enzyme system is a monooxygenase:
or often called hydroxylase: this is the most frequent reaction type that can be
reactions: epoxidation,
dehydrogenation,
oxidation to keto
group, aromatization)
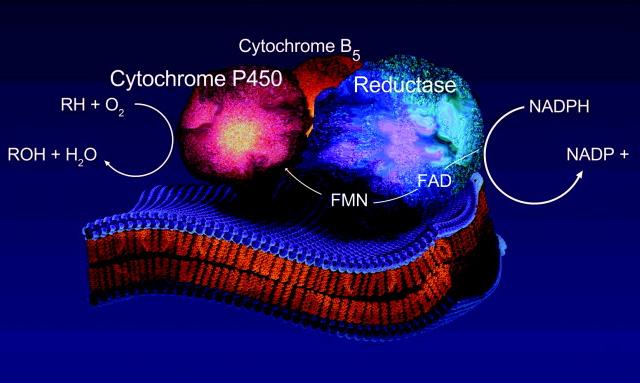
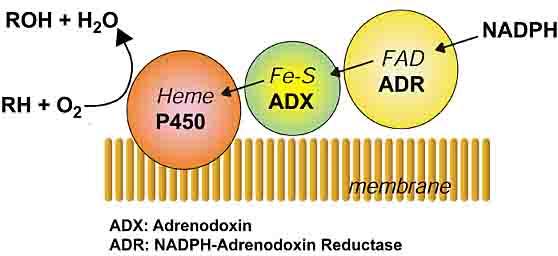
Components of ER electrone transport chain:
NADPH → cytochrome P450 reductase FAD, FMN → cyt. P450 isoenzyme NADH → citochrome b5 reductase FAD → cytochrome b5
cytochrome P450 reductase
Components in mitochondria: NADPH → ferredoxin reductase FAD → ferredoxin FeS → CYP isoenzyme
=adrenodoxin reductase
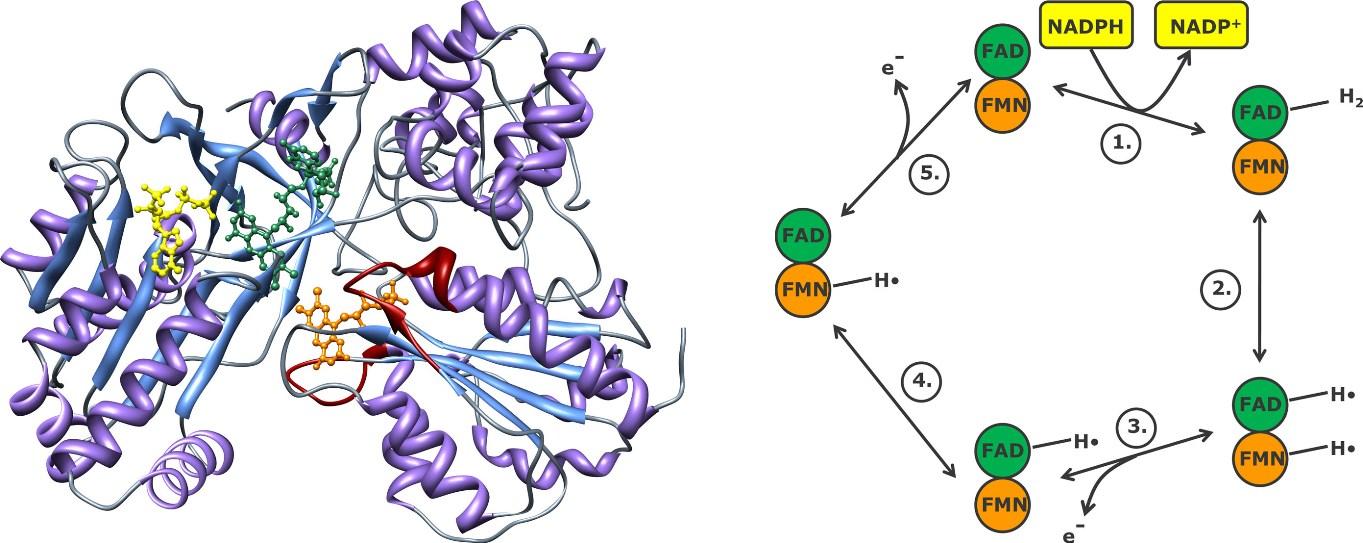
FAD and FMN prosthetic groups can add electrones in stepwise manner in cytochrome P450 reductase enzyme. Electons come from NADPH
Humans have 18 families of cytochrome P450 genes and 43 subfamilies
CYP1 drug metabolism (3 subfamilies, 3 genes, 1 pseudogene)
CYP2 drug and steroid metabolism (13 subfamilies, 16 genes, 16 pseudogenes)
CYP3 drug metabolism (1 subfamily, 4 genes, 2 pseudogenes)
CYP4 arachidonic acid or fatty acid metabolism (5 subfamilies, 11 genes, 10 pseudogenes)
CYP5 Thromboxane A2 synthase (1 subfamily, 1 gene)
CYP7A bile acid biosynthesis 7-alpha hydroxylase of steroid nucleus (1 subfamily member)
CYP7B brain specific form of 7-alpha hydroxylase (1 subfamily member)
CYP8A prostacyclin synthase (1 subfamily member)
CYP8B bile acid biosynthesis (1 subfamily member)
CYP11 steroid biosynthesis (2 subfamilies, 3 genes)
CYP17 steroid biosynthesis (1 subfamily, 1 gene) 17-alpha hydroxylase
CYP19 steroid biosynthesis (1 subfamily, 1 gene) aromatase forms estrogen
CYP20 Unknown function (1 subfamily, 1 gene)
CYP21 steroid biosynthesis (1 subfamily, 1 gene, 1 pseudogene)
CYP24 vitamin D degradation (1 subfamily, 1 gene)
CYP26A retinoic acid hydroxylase important in development (1 subfamily member)
CYP26B probable retinoic acid hydroxylase (1 subfamily member)
CYP26C probable retinoic acid hydroxylase (1 subfamily member)
CYP27A bile acid biosynthesis (1 subfamily member)
CYP27B Vitamin D3 1-alpha hydroxylase activates vitamin D3 (1 subfamily member)
CYP27C Unknown function (1 subfamily member)
CYP39 7 alpha hydroxylation of 24 hydroxy cholesterol (1 subfamily member)
CYP46 cholesterol 24-hydroxylase (1 subfamily member)
CYP51 cholesterol biosynthesis (1 subfamily, 1 gene, 3 pseudogenes)
lanosterol 14-alpha demethylase
Humans have 57 sequenced CYP genes and 58 pseudogenes.
only full length functional genes are named below
2A6, 2A7, 2A13, 2B6, 2C8, 2C9, 2C18, 2C19, 2D6, 2E1, 2F1, 2J2, 2R1, 2S1, 2U1, 2W1,
3A4, 3A5, 3A7, 3A43,
4A11, 4A22, 4B1, 4F2, 4F3, 4F8, 4F11, 4F12, 4F22, 4V2, 4X1, 4Z1
5A1, 7A1, 7B1, 8A1, 8B1, 11A1, 11B1, 11B2, 17, 19, 20, 21A2, 24,
26A1, 26B1, 26C1, 27A1, 27B1, 27C1, 39, 46, 51
Reactions of endogenous CYP substrates:
cholesterol and bile acids synthesis
CYP51= lanosterol-demethylase (ER)
squalene → squalene-epoxid →→ lanosterol → cholesterol
Synthesis of steroid hormons
"Old" name
Side-chain cleavage
enzyme; desmolase
3 β-hydroxysteroid
17 α-hydroxylase
11 β-hydroxylase
Author: R. A. Bowen
Study these structural formulas.
cholecalciferol =D3-vitamin → 25-OH-cholecalciferol → 1,25-dihydroxicholecalciferol=
kidney, bone, placenta calcitriol (hormon)
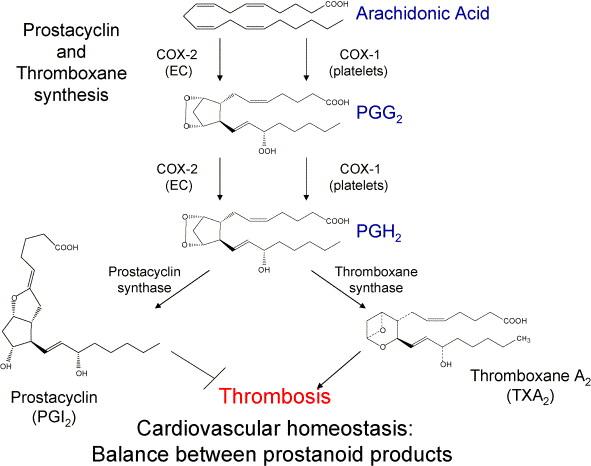
CYP4=desaturases
CYP8A1= prostacyclin synthase
linoleic ac. → arachidonic acid → prostaglandin H2 → prostacyclin
↓CYP5A1= thromboxane synthase
2nd phase: Conjugation reactions
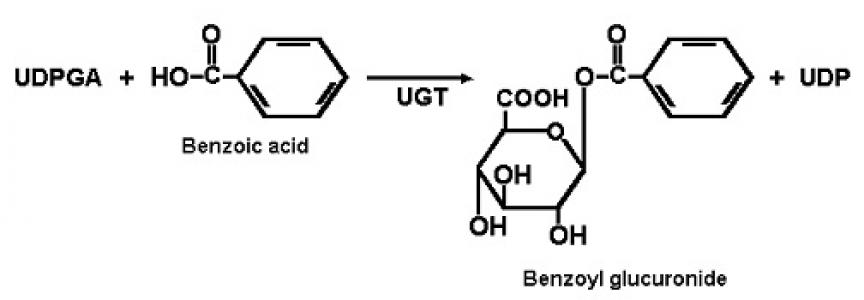
1.) glucuronidation by UDP-glucuronyl transferase
UDP-glucuronidate + drug = drug-glucuronidate + UDP
UGT1 forms by alternative splicing – bilirubin, amines, phenols are substrates
UGT2 formed by different genes – steroids, bile acids, opioids are substrates
Most frequent conjugation reaction. Enzymes in ER and cytoplasm of liver, skin,
breast, prostate, adipose tissue…
2.) Sulfatation by sulfotransferase
PAPS + drug = drug-sulfate + PAP
alcohols, phenols, arylamines are exogenious substrates,
steroids, heteropolysaccharides, glycolipids, glycoproteins, thyroid hormones
are endogenous substrates, the hormones are inactivated
in cytoplasm and Golgi apparate
3-4.) glutathion-conjugation by glutathione S-transferase
+ acetylation with acetyl-CoA by acetyltransferase
GSH + drug = drug-S-glutathione → Gly + Glu + drug-S-Cys → acetyl-cysteinyl-drug
slow and fast acetylators according to enzyme polymorphism
isoniazid, an antituberculotic agent can be toxic in slow acetylators
electrophylic SH-group
GSH = glutathione = γ-glutamyl-cysteinyl-glycine tripeptide Leukotrienes are formed by leukocytes, take part in inflammation.
5.) amino acid conjugation by: glycine, taurine
Benzoyl-CoA+ Gly = hippurate (way of elimination of N) + CoA
Phenylacetyl-CoA + Gln = phenylacetylglutamine (way of elimination of N) + CoA
chenodeoxycholate + taurine = taurochenodeoxycholate primary bile acid
cholate + Gly = glycocholate primary bile acid
6.) methylation by methyltransferase
dopamine + SAM = methyl-dopamine (inactive) + SAH
by catechol-oximethyltransferase = COMT in catecholamine degradation
noradrenalin + SAM = adrenalin + SAH
3rd phase: excretion of lipohilic and conjugated compounds
MDR(1-P) = multidrug resistance gene product and MRP (1-7) = multidrug resistance proteins are members of ABC (ATP-binding casette) transporters: when ATP is bound to their nucleotide binding domain the transporter opens and molecules are pumped out.
Induction, regulation of gene expression
Lipophilic compounds, hormons, foreign molecules (xenobiotics)
are ligands of their receptors that are actually transcription factor proteins,
which bind to promoter or enhancer element of the DNA and
ncrease transcription of mRNA, therefore from bigger amount of mRNA
bigger amount of proteins will be synthesized. This is the induction.
The opposite process is repression, when mRNA transcription is prevented.
Other mechanism: Hydrophilic second messengers e.g. cAMP, cGMP are
ligands of PKA and PKG kinases that phosphorylate and activate
transcription factors to translocate and bind to DNA etc.
Metabolism of vitamin D
1,25(OH) D is ligand of VDR transcription factor, it causes induction of many proteins.
CYP27B1:
1-hydroxylase
Metabolism of β-carotene and vitamin A
androgen receptor
estrogen rec. glucocorticoid rec. mineralocorticoid rec.
or steroid hormon or vit. D or A deriv. or bile acid or PUFA
NR = nuclear receptor = transcription factor protein that binds ligand
Transformation of medicines and xenobiotics
Drug metabolizing CYP enzymes 50 % - 3A4 20 % - 2D6 15 % - 2C9, 2C19 15 % - 1A2, 2A6, 2B6 …
regulation of enzyme synthesis is done by induction: inducer: drug, chemical, pollutants, contaminant, plant compound
inducer binds to any of the following transcriptional factors: CAR, PXR,VDR
that forms a heterodimer with another transcriptional factor: RXR
own ligand binds to RXR: retinic acid
enzyme protein synthesis
Transcription factors
from gene mRNA transcription
CAR= constitutive androstane receptor PXR= pregnane X receptor (inducers: phenobarbital, androstanol) VDR= vitamin D receptor (inducer: vit. D)
In mammals several transcription factor regulates the expression of the same gene
DNA chain HNF DR3 ER6 // ER6
xenobiotic responsive enhancer module
proximal promoter
HNF = hepatic nuclear factor
DR = direct repeat
RXR = retinoid X receptor
ER = everted repeat = inverse direction repeat of sequence
drug = medicine
VDR = vitamin D receptor
Substrates: these are degraded
Inhibitors
and compete for the enzyme
Amitriptyline* (Elavil)
Nefazodone > fluvoxamine (Luvox) > fluoxetine
(Prozac) > sertraline Inhibitors cause slower degradaton
Alprazolam (Xanax)
Paroxetine (Paxil)
of drug substrates, medicine
Triazolam (Halcion)
concentration remains high, can be toxic.
Midazolam (Versed)
Azole antifungals
Calcium blockers
Ketoconazole (Nizoral) > itraconazole (Sporanox)
Carbamazepine (Tegretol)
> fluconazole (Diflucan)
Cisapride (Propulsid)
Cimetidine (Tagamet)â
Dexamethasone (Decadron)
Clarithromycin (Biaxin)
Ethinyl estradiol (Estraderm,
Protease inhibitors
Glyburide (Glynase, Micronase)
Imipramine* (Tofranil)
Inducers
Inducer drugs cause faster metabolism of
Ketoconazole (Nizoral)
substrate medicines, so medicines will not be
Lovastatin (Mevacor)
effective enough, they do not reach the
Nefazodone (Serzone)
therapeutic concentration.
Terfenadine (Seldane)
Phenytoin (Dilantin)
Astemizole (Hismanal)
Rifampin (Rifadin, Rimactane)
Verapamil (Calan, Isoptin) Sertraline (Zoloft) Testosterone
Localization of CYP3A4
Venlafaxine (Effexor)
liver, GI: from esophagus till colon, resp. tract:
Protease inhibitors
nose and lung, kidney tubules, skin, blood cells,
Ritonavir (Norvir)
ovarium, testis Leydig-cells, adrenal gland zona
Saquinavir (Invirase)
glomerulosa, parathyroid gland, adenohypohysis
Indinavir (Crixivan) Nelfinavir (Viracept)
CYP2D6 SUBSTRATES AND
INHIBITORS
Inhibitors
Substrates
Paroxetine > fluoxetine >
Antidepressants*
sertraline (Zoloft) > fluvoxamine
Amitriptyline (Elavil)
Clomipramine (Anafranil)
Nefazodone (Serzone),
Desipramine (Norpramin)
Venlafaxine > clomipramine
Doxepin (Adapin, Sinequan)
(Anafranil) > amitriptyline
Fluoxetine (Prozac)
Cimetidine (Tagamet)
Imipramine (Tofranil)
Fluphenazine (Prolixin)
Nortriptyline (Pamelor)
Paroxetine (Paxil)
Venlafaxine (Effexor)
Haloperidol (Haldol) Perphenazine (Etrafon, Trilafon) Risperidone (Risperdal) Thioridazine (Mellaril)
Metoprolol (Lopressor) Penbutolol (Levatol) Propranolol (Inderal)* Timolol (Blocadren)
Codeine, tramadol (Ultram)
Metabolism of phenacetin and paracetamol/acetaminophen
acetaminophen ← phenacetin
In USA among all the acut liver failure causing effects (viruses, drugs, alcohol, toxins, hereditary illnesses etc.) alone acetaminophen is responsible for 1/3 of problems (50% in adults and 10% in children) The benzoquinonimin intermediate produced by CYP2E1 is very reactive: protein adduct is immunogenic, DNA adduct is mutagenic, reaction with PUFA in membranes causes lipid peroxidation and cell death. CYP2E1 is induced by alcohol and ethanol is the substrate of CYP2E1.
Oxidation of ethanol by ethanol-induced
In newborn, in fasting
Induced in alcoholists
and persons taking
certain drugs
Oxidation of alcohol is done by cytoplasmic alcohol dehydrogenase and peroxisomal catalase, but not induced.
According to the statistics of USA alone acetaminophen is responsible for the 1/3 of acut liver failure cases (50% in adults and 10% in children) and causes kidney failure too.
Acetaminophen =
paracetamol
ROS = reactive oxygen species (OH˙, O ˙-,H O )
are produced by CYP isoenzymes in ER, by mitochondrial electron transport chain, in peroxisome during FA oxidation and by xanthine oxidase, by NADPH oxidase in cytoplasm, spontaneously by metal ions. We have antioxidant, protective eznymes: superooxide dismutase, (SOD) catalase, glutathione peroxidase (GPX) thioredoxin (TRR) peroxiredoxin And by antioxidant compounds: ascorbate = vitamin C glutathione= GSH, vitamin E= α-tocoferol urate
Relationship between pesticide DDT, hormon
metabolism and BIOTRANSFORMATION enzymes
DDT = dichlordiphenyl-trichloretan is an insecticide. Insect-killing effect was discovered in 1934. In world war II the DDT was used against louse, flea and in tropic countries against malaria and yellow fever spreding mosquitoes, against thyphus, plague = pestilence, colorado-beetle. Rate of degradation of DDT in soil, water, plant, animal is 0-5% / year. It is accumulated in fat and milk. Most, but not all countries have withdrawn it from the market, now Mexico, China, India etc. produce it.
Europe and North America gets DDT back with Brasil crude coffee bean, African cocoa seeds and chocolate, Chineese peanut, Spanish and Greek etc. orange peel. Most polluted countries: Costa Rica, Zaire, India, Mexico, Pakistan, China
The effect of DDT in animals and human:
induces aromatase → testosteron is converted to estrogen in males
CYP2B and CYP3A enzymes are induced → testosteron hydroxylation and
inactivation is accelerated,
degradation of 70 % of medicines is increased, drugs have no effect
sulfotransferase enzyme is induced → testosteron and other steroid hormon
sulfatation and inactivation is faster
it has an androgen receptor antagonist effect
Because of above mentioned effects in
embryo, the inner gonad formation is
in adult males the androgen is inactivated,
the male is femininized, becomes impotent.
The similar dicofol, endosulfan and methoxychlor is used in USA,too.
CYP1A2 SUBSTRATES, INHIBITORS AND INDUCERS
Substrates
Smokers requier higher dose of
Amitriptyline* (Elavil)
theophylline and other drug
Clomipramine (Anafranil)* Clozapine (Clozaril)*
in war in VIETNAM 1962-1971 the herbicide
Imipramine (Tofranil)*
agent orange = phenoxy-acetate + TCCD
Propranolol (Inderal)*
as contaminant was used
R-warfarin* Theophylline*
TCCD is strong CYP1A inducer,
Tacrine (Cognex)
the strongest carcinogen
Inhibitors
Fluvoxamine (Luvox)
Grapefruit juice
Quinolones
R-Warfarin is an antithrombotic drug, a vitamin K
Ciprofloxacin (Cipro)
antagonist, that prevents formation of prothrombin
Enoxacin (Penetrex) > norfloxacin (Noroxin) >
in liver. If a CYPA2 inducer drug is added together
ofloxacin (Floxin) > lomefloxacin (Maxaquin)
with Warfarin, bigger amount of enzymes will fast
Inducers
degrade Warfarin, so the ineffective dose can not
Omeprazole (Prilosec)
prevent new thrombus formation.
If CYP2A2 inhibitor = repressor drug is added
Phenytoin (Dilantin)
together with Warfarin, it is not degraded, its
Rifampin (Rifadin, Rimactane)
concentration is toxically high, and in case of
hurt of the patient, the bleeding will not stop.
Charcoal-broiled meat* TCCD
Agent Orange
Agent Orange is the code name for one of the herbicides and defoliants used by the U.S. military as part of its herbicidal warfare program, Operation Ranch Hand, during the Vietnam War from 1961 to 1971. Vietnam estimates 400,000 people being killed or maimed, and 500,000 children born with birth defects. A 50:50 mixture of 2,4,5-T and 2,4-D, it was manufactured for the U.S. Department of Defense primarily by Monsanto Corporation and Dow Chemical. The 2,4,5-T used to produce Agent Orange was later discovered to be contaminated with 2,3,7,8-tetrachlorodibenzodioxin, an extremely toxic dioxin compound. It was given its name from the color of the orange-striped 55 US gallon (200 L) barrels in which it was shipped, and was by far the most widely used of the so-called "Rainbow Herbicides". During the Vietnam War, between 1962 and 1971, the United States military sprayed 12,000,000 US gallons (50,000,000 L) of chemical herbicides and defoliants in Vietnam, eastern Laos and parts of Cambodia, as part of Operation Ranch Hand. The program's goal was to defoliate forested and rural land, depriving guerrillas of cover; another goal was to induce forced draft urbanization, destroying the ability of peasants to support themselves in the countryside, and forcing them to flee to the U.S. dominated cities, thus depriving the guerrillas of their rural support base and food supply
180 millió $ kártérítés 45%-át a Monsato fizette a 210 000 veteránnak.
Viktor Juscsenko in 2004 and before
with metabolizable inducers:
with permanently present inducers (TCCD)
aromatic hydrocarbones
dietary plant constituents
tryptophan derivatives
TCCD = terachlorodibenzo-p-dioxin (inducers)
AhR = aromatic hydrocarbone receptor (transcription factor)
forms heterodimer with Arnt = aromatic hydrocarbon receptor nuclear translocator
AhR-Arnt translocation to nucleus and reacts with XREs =xenobiotic response elements =
AhREs = aromatic hydrocarbon responsive elements in the DNA
biotransformation enzymes induced
skin toxicity: chloracne (Viktor Juscsenko)
antioxidant enzymes induced
teratogenic response (abnormal emmbryo)
cdk inhibitors induced (cell cycle inhibited)
immunosuppression (low immune response)
proapoptotic Bax induced
IL-2 for T-cells
activation of MAPK cascade and
cell proliferation
abnormal hormon metabolism
neurotoxical effect
liver failure
cardiotoxic effect
Dohányfüstben is van
PAH = polyaromatic hydrocarbon
Enzymatic conversion of some selected human carcinogens towards their
ultimate DNA-reactive metabolites. Nature Reviews Cancer 5, 113-125
Conjugation sometimes causes activation:
*adrenal androgen dihidroepiandroszteron = DHEA + hydroxysteroid sulfotransferase
steroid target cell
dehidroepiandrosteron-sulfate actíve metabolite
Clinical aspects
*17-alfa-etinilestradiol anticontraceptive + rifampicin antituberkulotic HSST induktor
sulfonated and noneffective estrogen, pregnancy is possible
*bilirubin hem-degradative product in new born + not fully induced glucuronyl-transferase
bilirubin-diglucuronid is not produced, it can not be excreted, it accumulates in skin
in mucous membranes causing icterus = jaundice
Source: http://biochem-dnld.semmelweis.hu/ob_tanag_en/ob_elo_en/ob_elo_en_gy_02_02/g0202_en_MAT_20130514_biotransformation.pdf
Untitled
Orthodontic considerations for gingival health duringpregnancy: a review Authors' affiliations: Abstract: Gingivitis is caused by several known systemic Padma M. Mukherjee, Division of and local factors. Among systemic factors, the role of Orthodontics, Department of Craniofacial hormonal changes during pregnancy is well established. Sciences, University of Connecticut School
mecriticalcare.net
Recommendations for the diagnosis and management of corticosteroidinsufficiency in critically ill adult patients: Consensus statements from aninternational task force by the American College of Critical Care Medicine Paul E. Marik, MD, FCCM; Stephen M. Pastores, MD, FCCM; Djillali Annane, MD; G. Umberto Meduri, MD;Charles L. Sprung, MD, FCCM; Wiebke Arlt, MD; Didier Keh, MD; Josef Briegel, MD;Albertus Beishuizen, MD; Ioanna Dimopoulou, MD; Stylianos Tsagarakis, MD, PhD; Mervyn Singer, MD;George P. Chrousos, MD; Gary Zaloga, MD, FCCM; Faran Bokhari, MD, FACS; Michael Vogeser, MD