Angiogenic markers in breath condensate identify non-small cell lung cancer


ARTICLE IN PRESS
Lung Cancer xxx (2009) xxx–xxx
Contents lists available at
Angiogenic markers in breath condensate identify non-small cell lung cancer
C. Gessner , B. Rechner , S. Hammerschmidt , H. Kuhn , G. Hoheisel , U. Sack , P. Ruschpler , H. Wirtz
a Department of Respiratory Medicine, University of Leipzig, Liebigstrasse 20, 04103 Leipzig, Germanyb Institutes of Clinical Immunology and Transfusion Medicine, University of Leipzig, Johannisallee 30, 04103 Leipzig, Germany
Article history:
Early recognition of lung cancer is a prerequisite for any strategy to improve lung cancer treatment out-
Received 26 December 2008
come. Here we report a cross-sectional study intended as a proof of principle investigation using breath
Received in revised form 10 June 2009
based detection (exhaled breath condensate, EBC) of angiogenic markers (VEGF, bFGF, angiogenin), TNF-
Accepted 14 June 2009
␣ and IL-8 to discriminate 74 individuals, with confirmed presence or absence (X-ray, CT) of non-smalllung cancer (NSCLC). Levels of angiogenic markers bFGF, angiogenin and VEGF in EBC significantly dis-
criminated between 17 individuals with newly detected NSCLC versus stable and exacerbated chronic
Exhaled breath condensate
obstructive pulmonary disease (COPD) patients as well as healthy volunteers. Levels of IL-8 and TNF-␣ in
Multiplex bead based immunoassay
EBC indicated acute inflammation, e.g. in acute exacerbated COPD (AECOPD) and were not indicative of
Angiogenic markersLung cancer
lung cancer. In a different group of patients that were already treated with two cycles of chemotherapy
and who responded with at least a 25% reduction in primary tumor diameter, levels of angiogenic markers
were lower compared to patients with newly diagnosed NSCLC. We suggest that breath based detection
of angiogenic markers may help in the early detection of lung cancer.
2009 Published by Elsevier Ireland Ltd.
for the presence of lung cancer would be preferred over a sensitivetest for the presence of a nodule.
Three out of four patients with lung cancer are diagnosed
One approach to specific detection of lung cancer in the future
because symptoms of advanced disease are recognized. A practical,
may involve molecular markers ecent progress in understand-
non-invasive and relatively inexpensive method for the detection
ing tumor biology has facilitated the search for potential markers.
of lung cancer in a population at risk would be a prerequisite to
Several closely tumor associated markers have been identified and
improve the unfavorable outcome of this disease, which is the lead-
some of these may prove to be suitable for early tumor detection.
ing cause of cancer related death in the United States (Cancer facts
Molecular abnormalities such as mutations in the tumor suppres-
and Figures 2007, in American Cancer Society, 2007) ently
sor gene p53 tion or mutation of the retinoblastoma gene
early detection by screening for lung cancer is considered by using
ere detected and have been suggested to be potential markers
low dose CT and other means such as fluorescent bronchoscopy,
for the detection of lung cancer.
induced sputum or attempts to measure patterns of volatile organic
An important aspect of tumor biology is angiogenesis
compounds in the exhalate by one of several different methods
Tumors induce the generation of blood vessels that are neces-
addition to costs, radiation exposure or invasiveness (in
sary for further growth enic molecules have therefore
case of bronchoscopy), a significant effect of even the more thor-
been suggested as tumor biomarkers eased levels of vas-
oughly studied methods in improving outcome has not yet been
cular endothelial growth factor (VEGF) in serum and tumor tissue
demonstrated. Several large trials investigating the role of low dose
have already been correlated with poor prognosis in patients with
CT in lung cancer screening are ongoing throughout the world, but
lung cancer ly, increased levels of basic fibroblast
results will have to be awaited.
growth factor (bFGF), another potent angiogenic molecule, have
While looking for tumors in imaging tests may be quite sensitive,
been associated with poor outcome in lung cancer owever,
it will of cause never be highly specific. In theory, a sensitive test
these investigations used angiogenic growth factor levels in lungtissue of lung cancer patients which requires invasive diagnosticprocedures not suited for broad application.
Breath based methods may be a novel approach for identify-
∗ Corresponding author at: Department of Respiratory Medicine, University of
ing highly tumor-specific molecules in exhaled breath condensate
Leipzig, Liebigstrasse 20, 04103 Leipzig, Germany. Tel.: +49 341 971 2600;fax: +49 341 971 2609.
(EBC) may be used for selecting patients for further diag-
E-mail address: (H. Wirtz).
nostic work ups. We have previously demonstrated that detection
0169-5002/$ – see front matter 2009 Published by Elsevier Ireland Ltd.
Please cite this article in press as: Gessner C, et al. Angiogenic markers in breath condensate identify non-small cell lung cancer. LungCancer (2009),
ARTICLE IN PRESS
C. Gessner et al. / Lung Cancer xxx (2009) xxx–xxx
of p53 mutations in EBC of NSCLC patients is possible although
was based on criteria described by Anthonisen (presence of at least
quite time-consuming n this study we set out to investigate
one of the following three major symptoms: increase in dyspnoea,
the possibility of detecting more tumor-specific molecules in the
sputum volume increase, sputum change to purulence and at least
exhalate. We chose angiogenic molecules for their well-established
one of the following minor symptoms: cough, wheeze, sore throat,
association with tumor tissue as well as indicators of inflammation
nasal discharge, and fever). All AECOPD patients were treated with
in order to differentiate inflammation from tumor, e.g. in COPD.
oral corticosteroids and i.v. antibiotic therapy. All patients with
Detecting more than one marker in the small volume samples of
sCOPD, AECOPD, NSCLC and healthy individuals reported to be
exhaled breath condensate became possible using multiplex bead
non/ex-smokers for at least two years or smokers (current smokers
based immunoassays as previously reported in a study from this
and ex-smokers for up to one year) with comparable rates in all
group investigating cytokines in EBC of COPD patients
In this proof of principle study, we chose to investigate the fol-
Lung function (as performed the same day as EBC col-
lowing set of five markers in a customized array: three markers
lection in all stable COPD patients, tumor patients and volunteers
associated with angiogenesis (VEGF, bFGF, angiogenin) and two
and within one week in AECOPD patients. Capillary blood gas anal-
associated predominantly with inflammation, e.g. in COPD (TNF-
ysis was performed on all patients within the first couple of hours
␣, IL-8). These markers were tested in a group of individuals, of
whom 17 were just previously diagnosed with NSCLC while oth-
All COPD patients were in GOLD classes III or IV e
ers included exacerbated and stable COPD patients, and individuals
2006). None of the patients in this series was treated with oral
without lung disease.
steroids prior to admission. Instead all patients were on oraltheophylline and inhalation therapy (LABA and/or long acting
2. Materials and methods
anticholinergic and/or inhaled corticosteroid) according to GOLDrecommendations. All patients were regularly seen by a pulmonary
2.1. Study subjects and clinical scores
physician. Approval for this investigation was obtained from theethics committee of the University of Leipzig.
EBC was collected from 74 individuals (49 men, 25 women;
age: 61 ± 9 years): (a) healthy non-smoking individuals (volunteers,
2.2. EBC collection and markers
n = 12); (b) patients admitted for suspected lung cancer at the timeof confirmation of NSCLC (NSCLC; n = 17); (c) a different group of
EBC was collected for 20 min during regular breathing through
NSCLC patients with tumors in at least partial remission (i.e. reduc-
a mouth piece of the EcoScreen® (Jaeger/Cardinal Health, Hoech-
tion in primary tumor diameter of at least 25%) following two cycles
berg, Germany) while wearing a nose clip as previously described
of a platinum based chemotherapy (NSCLC-PR; n = 15); (d) patients
EBC samples were examined for amylase activity in order
with stable COPD (sCOPD; n = 15; stable COPD was defined by the
to exclude contamination by saliva (100 l of reconstituted EBC;
lack of symptoms typical for an acute exacerbation and no need
alpha-Amylase ESP1491300 kit; Boehringer Mannheim, Germany).
for a change in medication for at least eight weeks prior to pre-
EBC protein concentration was measured using the Micro-BCA-
sentation); (e) patients with exacerbated COPD according to the
Protein-Assay (Pierce, Rockford USA; detection limit: 0.5 g/ml).
Anthonisen criteria OPD; n = 15).
Patient characteristics are depicted in NSCLC was ver-
2.3. Lyophilization of EBC fluid
ified in all patients by histological examination of tumor biopsies.
EBC of patients with AECOPD was collected as early as possible and
Immediately upon collection, condensate samples were frozen
within 36 h of hospitalization. All AECOPD patients exhibited one
at −20 ◦C. A major portion of the samples (2 ml) used in the fluores-
or more criteria for admission to a hospital as suggested by Burge
cent bead array assay, was lyophilized on the evaporator (Uniequip,
and Wedzicha (respiratory rate > 25/min; pulse rate > 110/min;
Martinsried, Germany). The resulting pellet was resuspended in
PaO2 < 8 kPa; abnormal chest radiograph; serious concomitant dis-
60 l of ddH2O for direct use in the assay. This procedure resulted
ease; altered mental state; living alone Diagnosis of AECOPD
in a 33-fold concentration.
Protein in EBC (g/ml) (mean ±
All data are shown as mean ± S.D.
* p > 0.05 (no significant difference between investigated groups).
a Smoking was defined as current smokers or ex-smokers that discontinued smoking no longer than twelve month, non-smoking as no smoking longer than one year.
b Results were measured after AECB.
c Results were measured at time of hospitalization.
Please cite this article in press as: Gessner C, et al. Angiogenic markers in breath condensate identify non-small cell lung cancer. LungCancer (2009),
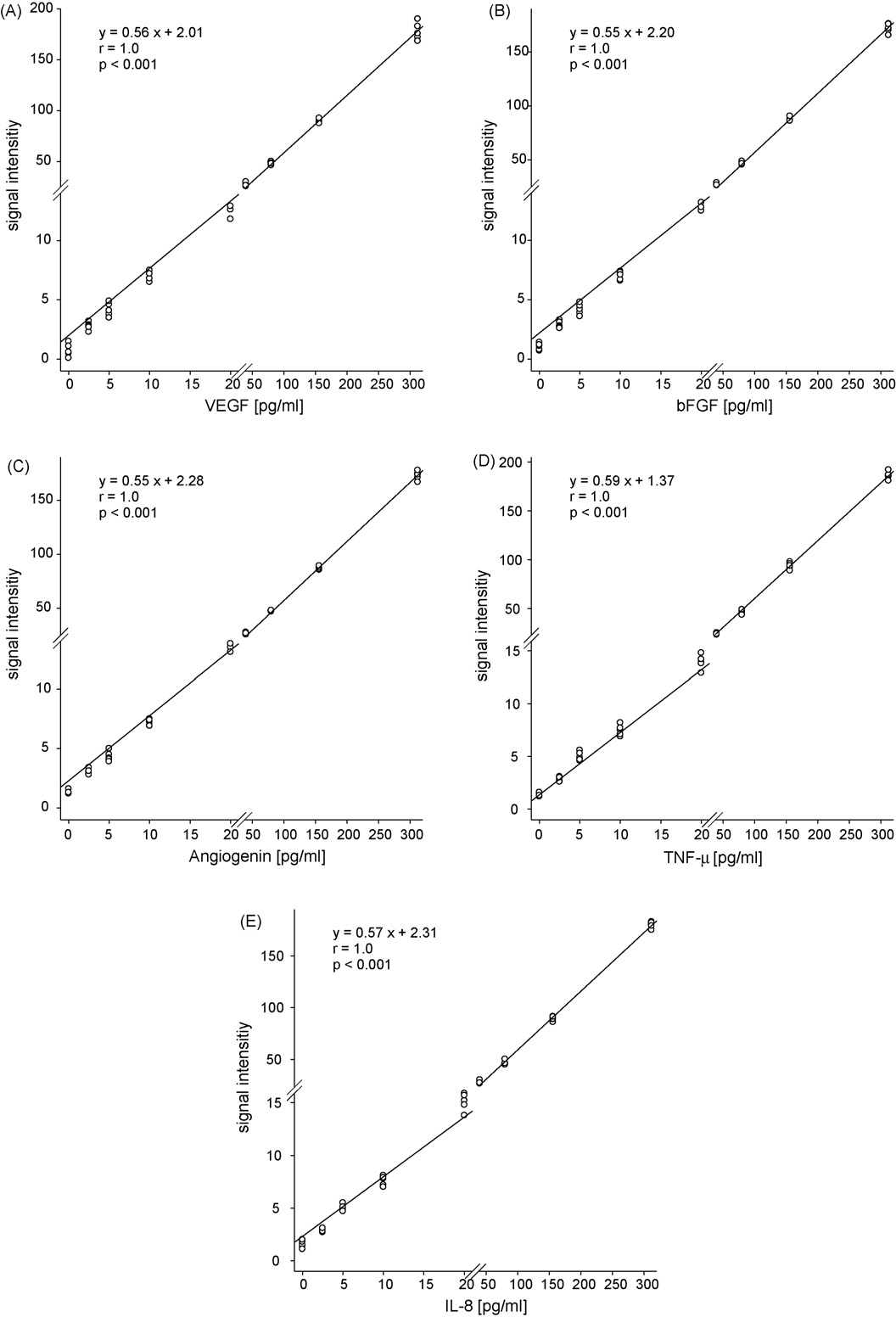
ARTICLE IN PRESS
C. Gessner et al. / Lung Cancer xxx (2009) xxx–xxx
Fig. 1. Calibration curves of VEGF (A), bFGF (B), angiogenin (C), TNF-␣ (D) and IL-8 (E) for EBC concentration ranges (2.5–312 pg/ml). For improved visualization of lower
concentrations, both axes were broken.
2.4. Multiplex bead based immunoassay
capture antibodies specific for angiogenin, bFGF, VEGF, IL-8,and TNF-␣ proteins were incubated with 1 ml of lyophilized
A multiplex fluorescent bead immunoassay (cytometric bead
breath condensate reconstituted with 60 l of ddH2O (dupli-
array, CBA; Becton-Dickinson, San Jose, CA, USA) was adapted
cate samples were prepared). Angiogenic factors in EBC samples
to exhaled breath condensate. A mixture of five bead popu-
and recombinant standards bound to capture beads were
lations with distinct fluorescence intensities and coated with
detected by phycoerythrin (PE)-conjugated detection antibodies
Please cite this article in press as: Gessner C, et al. Angiogenic markers in breath condensate identify non-small cell lung cancer. LungCancer (2009),
ARTICLE IN PRESS
C. Gessner et al. / Lung Cancer xxx (2009) xxx–xxx
Table 2
Recovery and variance of angiogenin, VEGF, bFGF, IL-8, and TNF-␣ in samples following spiking, lyophilization, reconstitution, and flow cytometric detection.
c (defined) (pg/ml)
c: concentration; S.D.: standard deviation; CI: confidence interval of mean; n = 3.
of equal specificity in a flow cytometer (FC500TM, Beckman Coul-
Total EBC protein concentration was measured from 100 l
aliquots in all unprocessed samples. Results are shown in There was no significant difference in any of the subgroups analyzed
2.5. Low concentration calibration of fluorescent bead array assay
(p = 0.27).
A calibration curve has been devised for the lower range of con-
3.2. Validation of the multiplex bead based immunoassay
centrations of angiogenic markers, IL-8 and TNF-␣ known frompilot experiments to be expected in exhaled breath condensate
The fluorescent bead array assay was validated in terms of recov-
A threshold concentration of 2.5 pg/ml was observed
ery and variance by measuring a known concentration of each of the
for reliable measurements. The calibration curve therefore ranged
angiogenic factors in 1 ml samples of a spiked reconstitution buffer
from 2.5 to 312 pg/ml. All data points were repeated five times.
following the procedure of freezing, lyophilization and reconstitu-tion. Three separate series of 6 concentrations (7.3, 14.7, 29.3, 58.7,
2.6. Statistical analysis
117.3, and 234.5 pg/ml) were prepared in triplicates and frozen at−20 ◦C. Test samples were then lyophilized using a vacuum con-
Statistical analysis was performed with the SPSS software pro-
centrator (Uniequip, Martinsried, Germany) and reconstituted at
gram (SPSS Inc., Chicago, USA). Linear regression analysis was
days one, seven and thirty. Data are shown in Recovery
applied to investigate the correlation of cytokine levels in EBC. Com-
of all markers and concentrations ranged from 86.4 to 104%. Previ-
parison of patient groups (three or more) was performed using the
ously the effects of lyophilization, reconstitution, and varying buffer
Kruskal–Wallis and Mann–Whitney U-tests. Statistical significance
composition of a different set of markers have been shown to be
was accepted at the 5% level.
3. Results
3.3. Angiogenic factors, TNF-˛, and IL-8 in EBC
3.1. Exhaled breath condensate characteristics
VEGF, angiogenin and bFGF were clearly elevated although
to varying degrees in patients with newly diagnosed lung
None of the condensate samples exhibited amylase concentra-
40 ± 10 pg/ml; angiogenin: 68.8 ± 32.8 pg/ml;
tions measurable with the assay used. Even though the detection
82.3 ± 34.4 pg/ml;
0.22 ± 0.24 pg/ml;
limit of the amylase assay may be higher than concentrations
0.34 ± 0.47 pg/ml) compared to stable COPD patients with-
expected in pure EBC relevant saliva contamination may
5.7 ± 5.5 pg/ml;
be excluded since amylase concentration in saliva is 10,000 times
4.2 ± 3.3 pg/ml; bFGF: 7.0 ± 5.1 pg/ml; TNF-␣: 0.16 ± 0.20 pg/ml;
higher than that in EBC.
IL-8: 0.29 ± 0.40 pg/ml) but also to all other groups and
Please cite this article in press as: Gessner C, et al. Angiogenic markers in breath condensate identify non-small cell lung cancer. LungCancer (2009),
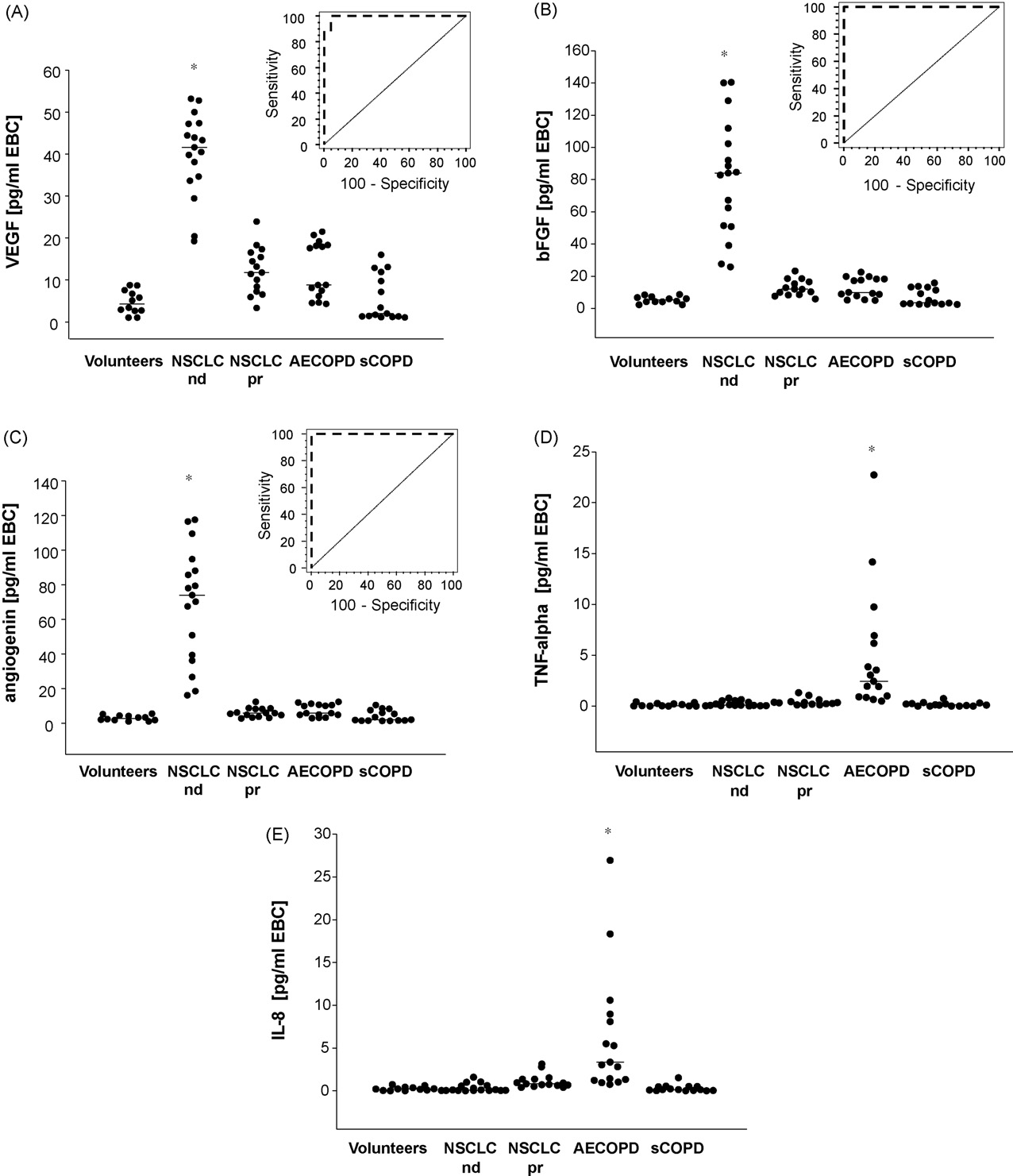
ARTICLE IN PRESS
C. Gessner et al. / Lung Cancer xxx (2009) xxx–xxx
Fig. 2. Concentrations of VEGF (A), bFGF (B), angiogenin (C), TNF-␣ (D) and IL-8 (E) in volunteers and patients with newly diagnosed NSCLC, NSCLC in partial remission,
AECOPD and stable COPD (pg/ml). Means are indicated by horizontal lines. Inserts in the upper right quadrants are receiver operating curves (ROCs) for VEGF (A), bFGF (B)
and angiogenin (C). Asterisks demonstrate significant difference of the marked group versus all other groups (p < 0.05).
Table 3
Patient's cytokine and angiogenetic levels.
IL-8 (pg/ml; mean ± S.D.)
n.d. (0.26 ± 0.23)
n.d. (0.29 ± 0.40)
n.d. (0.34 ± 0.47)
n.d. (1.12 ± 0.82)
TNF-␣ (pg/ml; mean ± S.D.)
n.d. (0.12 ± 0.15)
n.d. (0.16 ± 0.20)
n.d. (0.22 ± 0.24)
n.d. (0.41 ± 0.35)
VEGF (pg/ml; mean ± S.D.)
bFGF (pg/ml; mean ± S.D.)
Angiogenin (pg/ml; mean ± S.D.)
All data are shown as mean ± S.D.
Please cite this article in press as: Gessner C, et al. Angiogenic markers in breath condensate identify non-small cell lung cancer. LungCancer (2009),
ARTICLE IN PRESS
C. Gessner et al. / Lung Cancer xxx (2009) xxx–xxx
Table 4
Tumor parameters and correlation with VEGF, bFGF, angiogenin, IL-8, and TNF-␣ (ANOVA rank test [Kruskal–Wallis one-way analysis of variance on ranks] for three or more
groups, Mann–Whitney rank sum test for two groups).
T-stage of primary tumor
Lymph node metastases
Distant metastasis
Tumor stage groups
Squamous cell carcinoma
Large cell carcinoma
There was almost no overlap with any of the other groups
NSCLC histological subtype in this limited number of patients.
except for the two lowest individual values in the NSCLC group
Early diagnosis of NSCLC is highly desirable because early recog-
which were at a similar level as were the highest individual values
nition will allow detection of possibly curable disease. We are
in all of the other groups, and the AECOPD group specifically. We
however aware of the ongoing discussion on the general useful-
observed no influence of age, gender, tumor location or histology,
ness of lung cancer screening and the connected arguments both
tumor stage or TNM classifiers on the levels of VEGF and other
angiogenic factors in this limited number of patients (
A practical way of diagnosing lung cancer in the future might
In a different group of patients with NSCLC following two
be a two-step process that involves a test that is easily performed
courses of chemotherapy resulting in at least partial tumor
and very "cancer specific". This highly specific test would select
remission (>25% reduction in primary tumor diameter) sig-
patients, in whom CT imaging tests would have a high likelihood to
nificantly lower EBC levels of all angiogenic factors were
turn out positive. One advantage of this scenario could be a great
observed—comparable to the levels seen in patients with AECOPD
reduction of unnecessary diagnostic work ups due to "nodules".
(p < 0.0001). However, in these patients pro-inflammatory mark-
Another possible advantage might be improved patient acceptance
ers were somewhat elevated (p < 0.03). When levels of angiogenic
due to the fact, that a breath based test is easily performed within
molecules in EBC were analyzed for sensitivity and specificity using
the doctor's office, without the need of additional visits at a radiol-
receiver operating curves (ROCs) we observed excellent character-
ogist's office.
istics for all angiogenic molecules, with VEGF being just slightly
A breath based test however would have to be of great speci-
inferior compared to bFGF and angiogenin The ROC curve
ficity and sensitivity. This proof of concept study of detection of
analysis of patients with lung cancer compared to healthy con-
angiogenic molecules in exhaled breath condensate demonstrates
trols, stable and exacerbated COPD showed an area under the
that the method described is indeed highly specific and sensitive
curve for VEGF of 0.994 (standard error 0.013, p = 0.0001, sensitivity
in the relevant population (lung cancer versus COPD patients: both
100%, specificity 95.2%), for angiogenin of 1.0 (standard error 0.0,
current and ex-smokers). For the proof of concept purpose of this
p < 0.0001, sensitivity 100%, specificity 100%), and of 1.0 for bFGF
study it was necessary to investigate a group of patients with a
(standard error 0.0, p < 0.0001, sensitivity 100%, specificity 100%).
definite and verified diagnosis of NSCLC as early as possible fol-lowing verification. This meant examining patients at the time of
histological verified diagnosis. Due to this prerequisite we couldnot at this point test the detection of angiogenic molecules in EBC
In this study, levels of angiogenic markers in breath condensate
for the diagnosis of NSCLC at any time point earlier than that of
clearly differentiated between patients with NSCLC at the time of
the definite diagnosis of NSCLC as achieved by conventional diag-
histological confirmed diagnosis, and patients with either stable
nostic procedures. Our study is therefore limited to demonstrating
or exacerbated COPD, or healthy volunteers. There is no overlap in
the feasibility of a sensitive identification of lung cancer patients
angiogenic factors between patients with and without NSCLC for
among a relevant group of individuals with either known disease
angiogenin and bFGF and almost no overlap for VEGF. In contrast, a
(NSCLC) or individuals at risk (COPD) using the levels of angiogenic
great increase of inflammatory markers, IL-8 and TNF-␣, in EBC was
factors from breath condensate. Further validation of this method in
seen in exacerbated COPD albeit not in all cases. The increases of
the future will have to demonstrate the ability of detecting NSCLC
angiogenic markers did not correlate with the central or peripheral
in a previously undiagnosed population at risk. It will also have
localization of the tumor, or the T, N or M classification, or with
to be elucidated whether SCLC will also be detectable from angio-
Please cite this article in press as: Gessner C, et al. Angiogenic markers in breath condensate identify non-small cell lung cancer. LungCancer (2009),
ARTICLE IN PRESS
C. Gessner et al. / Lung Cancer xxx (2009) xxx–xxx
genic molecules in breath condensate. An additional limitation of
this study is the limited number of patients involved with very fewearly cancer stages.
In summary, EBC has potential to aid in the early diagnosis of
Adding the information provided by increased inflammatory
lung cancer A number of divers efforts have been reported
cytokines in EBC was useful for detecting patients with AECOPD
to detect lung cancer from exhaled air/breath condensate. They all
and may in rare cases increase the specificity of the test, but does
have their strong points and weaknesses. Sensitivity but also speci-
not appear to be necessary for the question of whether or not the
ficity appears to be of greatest importance to avoid anxiety and
patient is at high risk for having lung cancer. AECOPD is usually rec-
anger in potential patients. Our investigation demonstrates that
ognized easily by its clinical features. However a worsened cough
highly tumor-specific markers such as angiogenic factors measured
due to lung cancer in an outpatient in the pulmonary physician's
by sensitive assays in breath condensate might help to differenti-
office may occasionally be mistaken for AECOPD. The EBC based test
ate patients with and without NSCLC. This study will have to be
used in this study may then provide two rather specific indications
followed by a validation in a larger population.
leading to both directions by suggesting increased angiogenesis andthe lack of AECOPD typical inflammation.
Conflict of interest
Other authors have previously described EBC findings in lung
cancer patients. Carpagnano et al. reported increased IL-6 in a study
All authors declare that they have no financial or personal
of elderly NSCLC patients versus younger healthy controls
relationships with other people or organizations that could inap-
EBC IL-6 is also increased in other clinically relevant situations
propriately influence (bias) their work.
such as COPD and we and others have demonstrated this recentlyL-6 therefore does not seem to qualify as a highly tumor-specific marker in EBC. Similarly, endothelin was reported to be
increased considerably in EBC samples of NSCLC patients by the
[1] Hirsch FR, Merrick DT, Franklin WA. Role of biomarkers for early detection of
same group and may well be of greater specificity Several
lung cancer and chemoprevention. Eur Respir J 2002;19:1151–8.
lung diseases however also exhibit increased levels of endothe-
[2] Pastorino U. Early detection of lung cancer. Respiration 2006;73:5–13.
lin in the lung such as pulmonary hypertension and pulmonary
[3] Ravenel JG, Costello P, Silvestri GA. Screening for lung cancer. AJR Am J
fibrosis t is unclear however, whether endothelin levels are
[4] Conrad DH, Goyette J, Thomas PS. Proteomics as a method for early detection
increased in EBC in any of these diseases. DNA alterations mea-
of cancer: a review of proteomics, exhaled breath condensate, and lung cancer
sured from minute amounts of DNA in EBC have been demonstrated
screening. J Gen Intern Med 2008;23(Suppl. 1):78–84.
by our group in form of somatic mutations of p53 in a number
[5] Ahrendt SA, Hu Y, Buta M, McDermott MP, Benoit N, Yang SC, et al. p53 mutations
and survival in stage I non-small-cell lung cancer: results of a prospective study.
of lung cancer patients but not in controls In addition, the
J Natl Cancer Inst 2003;95:961–70.
group of Carpagnano et al. has described microsatellite instabil-
[6] Xu HJ, Quinlan DC, Davidson AG, Hu SX, Summers CL, Li J, et al. Altered
ity and loss of heterozygosity in smokers and in patients with
retinoblastoma protein expression and prognosis in early-stage non-small-celllung carcinoma. J Natl Cancer Inst 1994;86:695–9.
NSCLC hile the presence of NSCLC correlated to increased
[7] Kuhn H, Hammerschmidt S, Wirtz H. Targeting tumorangiogenesis in lung can-
microsatellite instability, a more severe smoking history did the
cer by suppression of VEGF and its receptor—results from clinical trials and
same. Thus microsatellite instability appears to reflect field cancer-
novel experimental approaches. Curr Med Chem 2007;14:3157–65.
[8] Folkman J. Is angiogenesis an organizing principle in biology and medicine? J
ization in the respiratory tract, but may not efficiently demarcate
Pediatr Surg 2007;42:1–11.
[9] Herbst RS, Onn A, Sandler A. Angiogenesis and lung cancer: prognostic and
Elevated VEGF in BALF of NSCLC patients compared to con-
therapeutic implications. J Clin Oncol 2005;23:3243–56.
[10] Fontanini G, Vignati S, Boldrini L, Chine S, Silvestri V, Lucchi M, et al. Vascular
trols has previously been reported by Ohta et al. Dalaveris
endothelial growth factor is associated with neovascularization and influ-
et al observed increased VEGF levels in serum but not in EBC
ences progression of non-small cell lung carcinoma. Clin Cancer Res 1997;3:
of lung cancer patients We cannot completely resolve this
incongruency of normal EBC VEGF levels in Dalaveris study and
[11] Fontanini G, Faviana P, Lucchi M, Boldrini L, Mussi A, Camacci T, et al. A high
vascular count and overexpression of vascular endothelial growth factor are
the significantly increased VEGF levels in NSCLC patients of our
associated with unfavourable prognosis in operated small cell lung carcinoma.
investigation. However, there are important differences in the two
Br J Cancer 2002;86:558–63.
studies: (a) the methods of measuring angiogenic molecules were
[12] Han H, Silverman JF, Santucci TS, Macherey RS, d'Amato TA, Tung MY, et al.
Vascular endothelial growth factor expression in stage I non-small cell lung
different; (b) Dalaveris et al. included both SCLC as well as NSCLC
cancer correlates with neoangiogenesis and a poor prognosis. Ann Surg Oncol
patients while our patient group was very homogenic with only
NSCLC patients; (c) Dalaveris and coauthors did not state whether
[13] Iwasaki A, Kuwahara M, Yoshinaga Y, Shirakusa T. Basic fibroblast growth fac-
tor (bFGF) and vascular endothelial growth factor (VEGF) levels, as prognostic
all patients included in their study were untreated at the time of
indicators in NSCLC. Eur J Cardiothorac Surg 2004;25:443–8.
breath condensate collection. Since we observed a great difference
[14] Chan HP, Lewis C, Thomas PS. Exhaled breath analysis: novel approach for early
in EBC levels of VEGF in patients being treated with chemother-
detection of lung cancer. Lung Cancer 2008.
[15] Gessner C, Kuhn H, Toepfer K, Hammerschmidt S, Schauer J, Wirtz H. Detection
apy (and responding), compared to other newly diagnosed NSCLC
of p53 gene mutations in exhaled breath condensate of non-small cell lung
patients, this might explain much of the difference.
cancer patients. Lung Cancer 2004;43:215–22.
TNF-␣ was increased in both EBC and serum of lung cancer
[16] Gessner C, Scheibe R, Wotzel M, Hammerschmidt S, Kuhn H, Engelmann L, et al.
Exhaled breath condensate cytokine patterns in chronic obstructive pulmonary
patients (SCLC and NSCLC) in the study by Dalaveris et al.
disease. Respir Med 2005;99:1229–40.
Similarly TNF-␣ in EBC was elevated in NSCLC patients in a study
[17] Sack U, Scheibe R, Wotzel M, Hammerschmidt S, Kuhn H, Emmrich F, et al.
of Carpagnano et al. However, considerable overlap existed
Multiplex analysis of cytokines in exhaled breath condensate. Cytometry A
between lung cancer patients and healthy controls for TNF-␣ levels
[18] Anthonisen NR, Manfreda J, Warren CP, Hershfield ES, Harding GK, Nelson NA.
in both serum and EBC. In our study, TNF-␣ was generally lower
Antibiotic therapy in exacerbations of chronic obstructive pulmonary disease.
compared to the study of Dalveris et al, and a trend for increased
Ann Intern Med 1987;106:196–204.
values in tumor patients compared to controls was observed, but
[19] Burge S, Wedzicha JA. COPD exacerbations: definitions and classifications. Eur
Respir J, Suppl 2003;41:46s–53s.
this effect was not significant. Again the reason for this difference
[20] Pauwels RA, Buist AS, Calverley PM, Jenkins CR, Hurd SS. Global strategy for the
remains unclear. In our study EBC was concentrated and TNF-␣
diagnosis, management, and prevention of chronic obstructive pulmonary dis-
was measured by a different technology (multiplex bead based
ease. NHLBI/WHO Global Initiative for Chronic Obstructive Lung Disease (GOLD)Workshop summary. Am J Respir Crit Care Med 2001;163:1256–76.
immunoassay). In AECOPD patients our test kit worked well in
[21] Gessner C, Kuhn H, Seyfarth HJ, Pankau H, Winkler J, Schauer J, et al. Factors
picking up greatly elevated EBC TNF-␣ levels.
influencing breath condensate volume. Pneumologie 2001;55:414–9.
Please cite this article in press as: Gessner C, et al. Angiogenic markers in breath condensate identify non-small cell lung cancer. LungCancer (2009),
ARTICLE IN PRESS
C. Gessner et al. / Lung Cancer xxx (2009) xxx–xxx
[22] Huszar E, Vass G, Vizi E, Csoma Z, Barat E, Molnar-Vilagos G, et al. Adenosine in
[29] Bacakoglu F, Atasever A, Ozhan MH, Gurgun C, Ozkilic H, Guzelant A. Plasma
exhaled breath condensate in healthy volunteers and in patients with asthma.
and bronchoalveolar lavage fluid levels of endothelin-1 in patients with
Eur Respir J 2002;20:1393–8.
chronic obstructive pulmonary disease and pulmonary hypertension. Respi-
[23] Effros RM, Hoagland KW, Bosbous M, Castillo D, Foss B, Dunning M, et al. Dilu-
tion of respiratory solutes in exhaled condensates. Am J Respir Crit Care Med
[30] Carpagnano GE, Foschino-Barbaro MP, Mule G, Resta O, Tommasi S, Mangia A,
et al. 3p microsatellite alterations in exhaled breath condensate from patients
[24] Goodwin M, Gleeson FV. The pitfalls of lung cancer screening. Cancer Imaging
with non-small cell lung cancer. Am J Respir Crit Care Med 2005;172:738–44.
[31] Ohta Y, Ohta N, Tamura M, Wu J, Tsunezuka Y, Oda M, et al. Vascular endothelial
[25] Gomez M, Silvestri GA. Lung cancer screening. Am J Med Sci 2008;335:46–50.
growth factor expression in airways of patients with lung cancer: a possi-
[26] Carpagnano GE, Resta O, Foschino-Barbaro MP, Gramiccioni E, Carpagnano F.
ble diagnostic tool of responsive angiogenic status on the host side. Chest
Interleukin-6 is increased in breath condensate of patients with non-small cell
lung cancer. Int J Biol Markers 2002;17:141–5.
[32] Dalaveris E, Kerenidi T, Katsabeki-Katsafli A, Kiropoulos T, Tanou K, Gourgou-
[27] Bucchioni E, Kharitonov SA, Allegra L, Barnes PJ. High levels of interleukin-
lianis KI, et al. VEGF, TNF-alpha and 8-isoprostane levels in exhaled breath
6 in the exhaled breath condensate of patients with COPD. Respir Med
condensate and serum of patients with lung cancer. Lung Cancer 2008.
[33] Carpagnano GE, Spanevello A, Curci C, Salerno F, Palladino GP, Resta O, et al. IL-2,
[28] Carpagnano GE, Foschino-Barbaro MP, Resta O, Gramiccioni E, Carpagnano F.
TNF-alpha, and leptin: local versus systemic concentrations in NSCLC patients.
Endothelin-1 is increased in the breath condensate of patients with non-small-
Oncol Res 2007;16:375–81.
cell lung cancer. Oncology 2004;66:180–4.
Please cite this article in press as: Gessner C, et al. Angiogenic markers in breath condensate identify non-small cell lung cancer. LungCancer (2009),
Source: http://www.becherconsult.eu/Lung_cancer_09_gessner.pdf
easymeasure.nl
Combining fluidized activated carbon with weak alternatingelectric fields for disinfection Justina Racyte , Jalal-Al-Din Sharabati , Astrid H. Paulitsch-Fuchs ,Doekle R. Yntema Mateo J.J. Mayer , Harry Bruning Huub H.M. Rijnaarts a Wetsus, Centre of Excellence for Sustainable Water Technology, Agora 1, P.O. Box 1113, 8900 CC Leeuwarden, The Netherlandsb Sub-Department of Environmental Technology, Wageningen University, Bornse Weilanden 9, 6708 WG Wageningen, The Netherlandsc Faculty of Chemistry, University Duisburg-Essen, Universita¨tsstraße 2, 45141 Essen, Germanyd EasyMeasure B.V., Breestraat 22, 3811 BJ Amersfoort, The Netherlands
wcauk.org
Achieving effective outcomes in patients with overgranulation Jackie Stephen-Haynes RGN DN DipH BSc (Hons) ANP. PG DipR PGDip Ed, Masters in Clinical Nursing Consultant Nurse and Senior Lecturer in Tissue Viability for Worcestershire Primary Care Trusts and University of Worcester. Stourport Health centre, Worcester St, Stouport on Severn, Worcestershire.DY13 8EH