Zdravotnipojistenci.cz

Tender systems for outpatient pharmaceuticals in
the European Union: Evidence from the
Netherlands and Germany
Panos Kanavos
with the assistance of Alessandra Ferrario Elena Nicod and Dale
Sandberg
LSE Health
London School of Economics
FOR THE EUROPEAN COMMISSION - DG ENTERPRISE
October 2012
Table of Contents
List of Tables and Figures
(a) Tables
Impact of the preference policy on prices of generic drugs in
the Netherlands, 2008-2012
Germany: Rebate contract awards by insurer, 2010
Market share and concentration levels in successive AOK
tenders, 2009 – 2013
Germany: Relationship between Patent expiry and tenders
issued, 2009-10
Overview of currently licensed biosimilar products in Europe,
Legal disputes for AOK Tenders, waves I – VI; 2009 - 2013
(b) Figures
Figure 1:
Germany: Development of generic market share for Cellcept, 7
months following its patent expiry (November 2010 –
February 2011)
Figure 2:
Assessment of discount contracts from the point of view of
AOK insures, 2009; N=2025; affirmative responses as % of
total responses
Appendix 1: Germany: Market share by % volume for Zyprexa 5 months
post patent expiry (September 2011) across three regions,
illustrating effect of presence or absence of rebate contract on
generic entry (October 2011-Feb. 2012).
List of abbreviations
AOK
Allgemeine Orts Krankenkasse
Central Nervous System
College voor zorgverzekeringen
Deutsche Angestellten-Krankenkasse
Economies of Scale
General Practitioner
Act Against Restraints on Competition (Germany)
Innungskrankenkasse
Intercontinental Medical Statistics
Royal Dutch Pharmaceutical Society
Over The Counter
Pharmaceutical Management Agency (New Zealand)
Stichting Farmaceutische Kengetallen (the Netherlands)
Social Health Insurance
Techniker Krankenkasse
Unive-VGZ-IZA-Trias
Wissenschaftliches Institut der AOK
Executive summary
Key trends from the implementation of tenders
The introduction of tenders/rebate contracts in the Netherlands and Germany over the
past 4-7 years has been characterized by fast uptake, increased implementation, and relative diversification in terms of the types of tendering models used. In the majority of
cases, the one-company-wins-all applies in both the Netherlands and Germany, whereas
in Germany, there may be up to three companies that are allowed to supply the market. In the Netherlands, the preference policy applies in parallel with other measures
implemented by health insurers, whereas in Germany, the rebate contracts apply to over 55% of the off-patent market and the rate is increasing. Tenders for out-patient pharmaceuticals present an attractive option for payers/health insurers because they are shown to drive prices close to marginal cost and, if sustained
over successive tender cycles, savings are achieved and retained. The submission of the tender implies that discounting practices at retail level are eliminated, and that, on
aggregate, health insurers are in complete control of the procurement process. Some of the stakeholders may be affected either because of greater logistics spending, discount
elimination and increased financial risks (pharmacy), or because of the requirement to explain to patients any change in the drug prescribed (both pharmacy and physician).
There is some confusion among patients if the drug changes at different intervals, but there is no evidence that this is creating problems with adherence or that it leads to
underuse or even, misuse of medicines. As a result of the increased implementation and fast uptake of tenders, the environment for manufacturers has become very challenging in a number of ways: first, prices have
been compressed down to marginal cost; second, due to price reductions and the increased implementation of the rebate contracts, changes in the business models and
the overall market structure have appeared. Third, several manufacturers have diversified their portfolio in order to stay on the market. Finally, regulatory, legal and
quality challenges remain.
Lessons from Germany
From a German specific perspective, there are significant challenges with implications
for health insurers, providers and other stakeholders. The first challenge is that there is
no publicly available information about winning prices, as these are commercial secrets and are part of the ownership of SHI funds. To that end, it cannot be ascertained what
the level of discount is. Challenges for providers relate to market uptake. Under the AOK tender variation, which
essentially allows only one manufacturer to win the contract in each lot, there is relative
uncertainty for the successful providers, as it is unclear what the implementation rate will be, due to the behaviour of prescribing physicians and the aut idem system. Additionally, awards within the AOK tender variation imply that the preferred product has a significant probability to be delivered more widely to all patients in general,
because of AOK's 41% nationwide market share and the resulting stocking preferences by pharmacies, which may be unwilling to hold another provider's drug in stock, partly
due to storage space restrictions. This is a challenge for competitors. Even under the variation that enables up to 3 providers to win contracts at national
level, there is still high uncertainty for successful providers, as it is still unclear which option the pharmacist will dispense. For instance, if one provider out of the three is well
established with (still) significant capacity to discount to pharmacy or able to market to physicians more aggressively, the pharmacist may dispense this product instead,
therefore well-established providers may obtain a (much) higher market share than the
(remaining) two less well known providers. A final challenge affecting all involved parties concerns competition. It appears that instead of intensifying competition, as desired, tendering has led to increase seller
concentration, particularly in that part of the system that is characterised by a single provider (the AOK variation) and that size of provider is in the majority of cases a
determining factor. It can be argued that tenders act as entry barriers to smaller firms. However, it is not known what impact this increase in concentration levels has had on
the winning prices over time.
Lessons from the Netherlands
From a Dutch perspective, the preference policy also presents challenges, despite the
low prices it has generated for health insurers.
The first challenge relates to how the preference policy is implemented and what impact
it is having on the pharmacy market. Pharmacist reimbursement and operating
peculiarities mean that reduced price margins directly impact on the financial context. Additionally, the pharmacist faces challenges in the functioning of the retail market and,
in particular, stock and logistics issues. Transaction costs and stocking issues for
pharmacies can increase considerably as different insurers have different preferred drugs and if a pharmacy does not have a preferred drug it potentially violates its
contract with the insurance in question. The second challenge is the lack of availability for a number of products on the Dutch
market. Inadequate lead-times between announcing winners and the implementation of the preference policy results in supply gaps. Secondly, despite the pledge by
manufacturers to provide a continuous supply over the contract period supply challenges throughout the preference period suggest that some are unable to meet the
required volumes. The lack of availability is therefore pervasive at facility level. Stock problems at the facility level are augmented by the difficulties that pharmacists may face
in sourcing preferred products, particularly for smaller patient groups. Finally, the fourth challenge relates to the multiplicity of schemes applying to the
reimbursement of generic medicines in the Dutch market. From a provider's perspective, avoiding a monopsony environment is good. It can be argued that the other
segments (the Uvit system and the IDEA model) are used to cross-subsidise
manufacturers who would otherwise make a loss in the preference system. From a pharmacist's perspective, however, managing multiple purchaser contracts, carries
financial risks and administrative costs.
Common practical challenges of tenders
Regulatory compliance is intense, requires significant attention and its costs should not
be underestimated. The European procurement law is often perceived to be very
formalistic and requires detailed information by those who submit offers; companies can be disqualified if they do not submit the right information to the authorities (e.g.
during interviews it was mentioned that a company was disqualified because the electronic signature was not the right one). In addition, all steps into the system have to
be confirmed and this relates to all parts of the supply chain to ensure that they can deliver the product(s) for the 2-year duration of the contract, even in the cases of sub-
contracting.
Quality assurance might be at stake if companies always need to supply at the lowest
possible price. Many manufacturers are trying to shift supply across their affiliate
population, or by sub-contracting within or outside Europe, including India and China. Ultimately, however, the winner(s) of the contract is (are) responsible for safeguarding
quality and the penalties for not doing so can be significant, in addition to any
replacement costs.
There are also legal challenges, which essentially highlight the fact that the legal
ramifications in this part of the system may still need to be further clarified, despite the clarity surrounding the EU procurement directive. One such issue has arisen in
Germany, where the right to award the tender by a sickness fund (BKK) to three providers has been challenged on the grounds that two of the "preferred" manufacturers
have large sales forces which can be perceived as unfair competition. In the Netherlands a legal dispute between GSK and Teva illustrates the evolving
competition concerns in the generic market. Generic suppliers cannot submit a price to the taxe prior to the expiry of patented products since this is considered a commercial
act. This leads to delays of on average six weeks before generic products enter the market, decreasing the chance of being selected as a preferred product for the
subsequent period, as well as increasing the risk of litigation in a low-profit margin environment. Although this does not affect the preference policy per se, it becomes less
attractive and risker for generic companies to introduce new products, potentially
reducing competition.
Longer tender periods may lead to the potential discontinuity of a selling product that
has lost its preferred status; for instance, manufacturers that are not preferred have an incentive to stock-out or even exit the market. Particularly where the tender makes up a
large portion of the market, it is very difficult for suppliers to remain active. The lead times in order to re-stock are approximately 6-7 months and this can create an issue if
preferred manufacturers face an unexpected supply problem.
Availability problems and supply shortages were discussed above, in relation to the
Dutch system. They may be due to two interlinked reasons: first, due to the short period between acceptance of the bid and start of the rebate contract or the preference period.
Stock-filling lead times required by manufacturers in both countries are in general 6-7 months or more depending on volumes to be supplied. Supply defaults during
contracted periods also lead to challenges beyond the initial ‘teething' problems. Although tender awards are usually accompanied with guarantees on supply, there have
been circumstances where manufacturers were unable to supply agreed-upon
quantities. This becomes difficult if the entire market is subject to the same scheme and
contingency plans must be in place.
Timing of a tender immediately after the expiry of the patent of a molecule may have
significant implications for competition. The challenge from a supplier's perspective in
this context is twofold: first, suppliers entering the market late cannot participate in the tendering process and are altogether excluded from the market for this product. Second,
if all insurers issue tenders immediately after the expiry of the patent of the originator molecule, then there is practically no market potential for newly genericised molecules
outside the tender market. Both these aspects may have an impact on the amplitude and
extent of competition that takes place following genericisation.
The potential for biosimilars remains largely unrealised and the view is that physicians
need to be involved in decisions about which product patients ought to receive. Starting
a therapy on a new patient seems to be less contestable and this presents opportunities
– currently – for biosimilars.
Prices achieved and savings to health insurance
The prices achieved in both the Netherlands and Germany on molecules tendered are
very favourable to health insurers and sickness funds. The evidence from the
Netherlands suggests that since genericised molecules have been subjected to tendering, the price levels achieved are consistently low over several tender cycles compared with
the price of the molecule at patent expiry. The discount off the price at patent expiry
exceeds on many occasions 93% to 95%. Publicly available information on prices achieved is not available in Germany, although interviews with stakeholders suggest
that they lie within the same or a comparable range (ie exceed 90% discount off the price of the molecule at patent expiry). The savings achieved by procuring off-patent medicines through tenders can be significant and have been shown to run up to Euro 1 billion in Germany. Although this is
a one-off saving for the relevant market segment, sustaining it implies that the same or
comparable price levels are achieved over successive tender cycles
Tendering in an environment of multiple insurers
Tendering practices in Germany and the Netherlands are now extensively used by
different sickness funds (Germany) or insurers (the Netherlands), but are used in parallel with other policies and are not the sole way of procuring generic medicines.
This has important policy implications for countries whose health care systems are characterised by a single purchaser. If a tender policy is introduced around the lowest-
price-wins-all principle and with a minimum duration of 1 year, then implementation of
a single tender per molecule in a monopsony environment might have short-lived effects for the monopsony, as the market will be effectively closed to all other
competitors, who may exit and find it difficult to re-enter. The wider implementation of tendering practices in systems characterised by monopsonistic structures, therefore,
needs to be carefully thought and organised in ways that would safeguard opportunities for as many players to compete on the market at as many points in time as is feasible.
Although the existence of multiple markets enables suppliers to cross-subsidise across markets, if a product struggles in one market, it can be difficult to shuffle between
markets and stakeholders highlighted the issue of stock write-off at provider and pharmacy levels when stock could not be shifted. In the context of multiple insurers
with changing tender periods, new products and different packaging requirements,
leaflets etc. make shifting excess stock difficult.
Impact on generic market structure and future competition
Evidence is beginning to emerge that the wider implementation of tendering practices in
Germany and the Netherlands has begun to affect the generic industry market structure:
some manufacturers are no longer participating in tenders and have downsized their operations already; others have changed their business model and established
subsidiaries that are only dealing with tenders, with zero sales forces and reliance on sub-contractors to deliver product; finally, most manufacturers are seeking
opportunities to diversify their business portfolio by identifying niche markets (e.g. women's health; special therapeutic areas which are not necessarily subjected to
tenders, OTCs).
Industrial policy for the generics sector
The changing environment and market structure raises the question of whether there
needs to be an industrial policy consideration for the European generics business. The
evidence suggests that health insurers and sickness funds are not making contract decisions based on industrial policy considerations, but on the grounds of the cost of
medicines they procure. While this is understandable, it also raises questions about the overall survival of the generics sector in Europe. This may be an undue concern as
generic manufacturers are clearly taking steps by diversifying their portfolios and
business models to the new reality, but it begs the question of whether a wider reflection might need to take place in this regard, and what role of the European
Commission might play in this regard.
Impact on other stakeholders
Overall, important lessons can be learned from the experience of tenders to date. While
the economic rationale of tenders is undisputed, the likely impact on other stakeholders
needs to be taken into consideration and minimised. Patients have on certain occasions expressed concern and confusion about a medicine being changed to one that has
different shape or colour. This may be of importance to elderly patients with multiple co-morbidities who are receiving multiple medications. All the above might have an
impact on patient adherence to treatment, although hard data to support this is not available. The solution to this problem is continued information from health insurers
and greater interaction with pharmacists. In both Germany and the Netherlands, tender policies have increased transaction costs and the costs of procurement and stocking for
pharmacists. Reaction to the policy has been greatest in the Netherlands due to the impact tendering had on pharmacy income compared with Germany where income
remained unaffected. Physicians also need to be in a position to explain changes in the
product supplied to their patients and this can be time consuming.
Sustainability over the longer term
As far as payers/insurers are concerned, the strategy will always be to obtain the best
possible price for products which are genericised on the understanding that quality and
continuity of supply can be guaranteed and maintained. Dutch insurers (subscribing to the preference policy) and German sickness funds (aggressively pursuing rebate
contracts) have shown they are not prepared to pay any premium for off-patent medicines. For generic manufacturers, it is clear that the operating environment in Europe is becoming increasingly challenging. In order to deliver – in a sustainable manner - the
prices that health insurers have got used to over the past few years, it looks as though changes in market structure and manufacturers' business model are inevitable. Spare
capacity that can deliver these prices can be found either through sub-contracting elsewhere in Europe, or, more realistically, outside Europe. The latter carries with it a
quality risk, despite manufacturers' willingness to guarantee quality since control is more fragmented. Overall, it appears that the long-term implications of lower prices
achieved through tendering practices will be a (further) contraction of generic
manufacturing and marketing activities in Europe and a shift of manufacturing outside Europe. An additional dimension in this context – whose impact is unknown and unpredictable at the moment - is the potential desire by other EU Member States to implement
comparable practices for procuring (off-patent) medicines in their outpatient markets. The more countries decide to do so, the faster the precipitation to an environment
whereby a European generics industry will cease to exist in its current form.
1. Background
Health purchasers are increasingly preoccupied with lowering prices of pharmaceuticals as well as overall pharmaceutical expenditure. Different policies have been implemented to achieve this, one of which is tendering for outpatient pharmaceuticals.
Tendering may take different forms determining the drugs that are selected (i.e. generics, or branded): 1) a molecule-based tender targets bio-equivalent pharmaceuticals, which are highly substitutable medicines most often from the off-patent market; 2) a therapy-based tender applies to all products in a same therapeutic class which may include in some cases branded drugs; and 3) portfolio contracts, whereby products are grouped and rebates apply for that group of products. The objective of tendering is to ensure that the necessary pharmaceuticals are available in the required quantities, at reasonable prices and at a recognised quality standard. The rationale for implementing such tools is, first, to stimulate price competition, and achieve the lowest prices for health insurers; second, to ensure transparency in procurement procedures; and third, to redistribute any discounts captured by the distribution chain to health insurers.
In 2009, EMINet produced a report1 on tendering comparing the scheme in the Netherlands (preference-based policy), with the cases of Germany and Belgium. This report focused mainly on the consequences of tenders in outpatient pharmaceuticals, for which little quantitative evidence was available, and discussed the likely implications of tendering on key stakeholders, notably sickness funds, patients, physicians, pharmacies, the generic industry, and the originator-brand industry.
The main effect of tendering in Germany and in the Netherlands was seen to be a shift in the balance of power in favour of insurers (i.e. sickness funds and health insurance respectively), whose main objective is to generate additional savings. As a result, the report suggested that in the short term, the prices of tendered pharmaceuticals dropped and resulted in substantial cost-savings (eg. € 355 million in the Netherlands in 2008); however, reported figures were revised downwards to account for increases in distribution and other costs.
While the net effect of outpatient tender practices in the Netherlands and Germany was deemed to be positive for the payor community, there was uncertainty as to whether it would outweigh other indirect and administrative costs generated from this scheme. This report endeavours to address these uncertainties and attempts to estimate the cost savings to health insurance or sickness funds and the overall implications of introducing tendering practices based on data and evidence which spans a longer time period. 1 Kanavos, P., Seeley, E., and Vandoros, S. October 2009. "Tender systems for outpatient pharmaceuticals in
the European Union: evidence from the Netherlands, Germany and Belgium". EMINet.
2. The research questions and objectives
The results of the tendering process, as related to prices achieved and their long-term sustainability, was one of the main issues raised in the 2009 EMINet report. A key question was whether manufacturers would be able, in the long run, to continue to offer such low prices. If this is not the case the risk is that the number of players on the market may decline, resulting in less competition and, possibly, higher prices. Nevertheless, the effects of competition on firms are still widely unknown. This study attempts to ascertain the performance of tendering schemes and their effect on competition, notably for a longer period of time and by focusing on different molecule areas.
Another recurrent question is whether health insurance companies or sickness funds violate competition rules, by abusing of their dominant position. This was particularly an issue in the German rebate system. This study will also include any advances on the legal front for the German rebate system.
Up to now, no negative effects from tendering on the access to medicines have been noticed, other than transient effects due to some players' inability to service the market, which were dealt with swiftly. Continuity of treatment was considered to be more of a challenge. That is, when a patient has been on a particular treatment for a period of time this will be disrupted if an alternate provider wins the relevant tender. This may be most likely to occur in therapeutic-based tendering, and may result in psychological effects or have an impact on adherence to treatment. This study addresses this issue by discussing the extent to which decision-makers have made any adaptations that have any measurable effect on the above.
Overall, the 2009 report contained good qualitative evidence but not enough quantitative evidence on tendering, mainly on its long term implications (i.e. competition and prices, among others), their impact on the stakeholder community (i.e. on the generic industry or pharmacies in the long run), nor address the differences in the implications of molecule-based and therapeutic-based tendering. Therefore, a specific aim in the current study is to include as much quantitative evidence as possible and available.
In light of the above, the objective of this study is to attempt an evaluation of the tendering schemes in Germany and the Netherlands, by drawing on a wide range of external stakeholders, whilst at the same time pursuing quantitative data (e.g. on prices, volumes, spending and other variables), which were not available last time round.
3. Methods
This report focuses on two study countries, the Netherlands and Germany, on the basis that they have comparable tendering systems that apply to ambulatory care, and where, in principle, the lowest price wins the bid. The analysis in this study relied on primary and secondary data collection and was completed in three phases.
During the first phase, secondary collection and review was performed, concerning mainly the period between 2008 and the 2011, since the period before 2008 was already covered in the 2008 EMINet report on tendering. Data sources comprised a systematic review of the available (peer review and grey) literature. The systematic literature review of peer-reviewed and grey literature on the impact of tendering systems on manufacturers, payers, and patients was undertaken in March 2011 and was updated in December 2011. In terms of keywords, the following search strategy was used ("rebate contracts" OR "tendering") AND ("pharmaceuticals" OR "drugs" OR "medicines") in PubMed, Web of Knowledge, and Scopus. For the Google search the following key words were used ("rebate contracts" OR "tendering" OR "preference policy"2 OR "rabattvertäge"1 OR "rabattvertraege" 1) AND ("pharmaceuticals" OR "drugs" OR "medicines").
A special focus on the eventual changes in pharmaceutical policy in the study countries that occurred in this short period also took place, including advances in the legal framework and related jurisprudence, where necessary. This phase was also used to structure an interview guide to be used in semi-structured interviews with the relevant stakeholders in phase 2. The interview guide focused on issues concerning impact assessment with particular focus on prices of and savings from generic medicines that were tendered, competition aspects, market structure, legal aspects, biosimilars and tenders, product supply and shortages, and evidence of impact on health care professionals and patients.
The second phase consisted of material collected from semi-structured face-to-face or telephone interviews with stakeholders in the Netherlands and in Germany. The stakeholders encompassed those who have already contributed to the 2009 report, as well as others. Stakeholders included policy-makers, health insurance representatives, and representatives from the wholesale/retail associations, representatives from the pharmaceutical industry (generic and research-based), physicians and patients. A total of N=34 individuals were interviewed in this context. Fieldwork took place between May and November 2011, over a series of face-to-face meetings and telephone interviews.
During the third phase, the quantitative data collected were analysed. Attempts were made to conduct the analysis based on a number of endpoints as follows: 2 These key words were omitted from the searches in PubMed, Web of Knowledge, and Scopus because they did not lead to any results.
estimation of cost savings from tendering, based on the data on prices of tendered (NL) or rebated (D) medicines; legal implications and their effect on the tendering policy from the Dutch and German systems, including any advances on this legal front in the last two years; competition issues; product supply and likely shortages; and biosimilars.
4. Results of the literature search
The peer-reviewed search originally retrieved 82 articles. Sixty-one and further 13 papers were excluded by title and abstract screening respectively. Papers were excluded either because they were not relevant to "tendering of outpatient pharmaceuticals" or because they were discussing tendering in low- and middle-income countries or because they were discussing issues potentially related to tendering but were not relevant in documenting the impact of tendering on manufacturers, payers, and patients. Following exclusion, eight articles were retained (Blackmore, 2005; Carradinha, 2009; Dylst, Vulto, & Simoens, 2011; Hoffmann, Glaeske, & Pfannkuche, 2009; Kostev, Fuchs, Bauer, & May, 2011; MacKay, 2005; Natz, 2008; VanHaeren, Arickx, Soete, & Bormans, 2009) Additional references were identified through the reference lists of the selected studies (Cheraghi, 2009; Institut für Demoskopie Allensbach, 2009; Rücker, 2008).
Grey literature search through Google led to the identification of a further three articles from Germany (Gröber-Grätz & Gulich, 2010; May, Kötting, & Cheraghi, 2010; Quinzler, Bertsche, Szecsenyi, & Haefeli, 2008). Finally, two additional reports known by the authors to contain information on savings from tendering were added (Foundation for Pharmaceutical Statistics 2010; Pharmaceutical Management Agency 2011)
4.1. Literature review on tendering for out-patient medicines
Evidence from a recent survey on tendering for outpatient drugs suggests that the latter is more prevalent in countries with a developed generic market. The study found that only 12.5% of the countries with a developing generic market3 were using tendering for out-patient medicines in comparison to 54% of the countries with a developed generic market among in nineteen EU countries (Dylst et al., 2011).
A review on the short-time impact of pricing policies highlights a number of issues for patients, government and manufacturers. These include supply issues; disincentives for wholesalers and pharmacists because of lower profits and increased risk they are asked to assume in terms of maintaining stocks; 3 In this study, a generic medicines market was considered to be mature when the market share of generics by volume exceeded 40% whilst developing markets had a market share of generics by volume below 25%.
availability of generic medicines in countries using tendering; sustainability for the generic industry; disincentives for innovation; administrative burden for the actors involved; regulatory issues leading to marketing delays and the threat that some products that fail to win tenders for three consecutive years will lose their license because of the sunset clause (Carradinha, 2009).
4.2. Evidence on the impact of rebate contracts in Germany
4.2.1 Impact on patients
Rebate contracts in Germany cause patients to switch between products. A survey of 2,500 patients aged 16 and more revealed that 7% of all the interviewees experienced problems of tolerance and side-effects when switching from one product to another (Institut für Demoskopie Allensbach, 2009). In the elderly this percentage was 11% (Institut für Demoskopie Allensbach, 2009).
In a survey of people aged 60 and over more than 25% of the participants complaint of constantly being dispensed different products from different manufacturers, having received medicines they did not tolerate well (more than 30%), being confused about which the indication of their medicines (more than 40%), having had arguments at the pharmacy (more than 40%), having the feel of being given the wrong medicine (more than 25%), having experienced side-effect (about 5%), and only 10% of the participants reported not having experienced any problem as a consequence of medicine switching with the rebate contract policy (Cheraghi, 2009).
Compliance problems were highlighted in a survey of 95 general practitioners (GPs) in Germany. In this study 87.4% of the interviewed GPs reported problems of patient compliance due to patients being switched to another drug as part of the discount contracts (Gröber-Grätz & Gulich, 2010). Further consequences as reported by GPs include, negative consequences on the patient-doctor relationship (73.4%), need for increased patient counselling (90.5%), and the threat of medication errors caused by switching treatment (73.4%) (Gröber-Grätz & Gulich, 2010). On the physician's side it unveiled information problems as doctors are often unclear which health insurer contracts with which manufacturer and for which drug (Gröber-Grätz & Gulich, 2010).
An investigation of the negative impact of rebate contracts on patients with depression showed decreased compliance for patients who are switched to an alternative drug (27.7% patients stopped treatment 3 months after being switched to an alternative treatment in comparison to 18.7% patients stopping treatment within the same time period but continuing with their initial medication, p<0.0001) (Kostev et al., 2011). Large differences were also observed in hospitalisation rates and resource utilisations. The risk of hospitalisation was 18% higher (adjusted for demographic and clinical
variables) for patient who had been switched to an alternative drug and 22% higher for patients (>65 years old)(Kostev et al. 2011). The estimated direct costs of hospitalisation due to treatment switch were €19.9 million per year while the indirect costs were estimated at €3.5 million per year (Kostev et al., 2011).
Another study from Germany looked at the practical implications for patients switching drugs as part of the rebate contracts policy. In 10% of the substitution cases analysed (N=182), patients requiring to take a smaller dose than the dispensed form, were switched to a formulation (e.g. unscored tablet, capsule) which did not allow splitting (Quinzler et al., 2008).
A study on the consequences of the introduction of rebate agreements on the non-substitution option showed an increased use of the non-substitution option for prescriptions between 2006 and 2008, geographical variations, and a higher use of this option in the elderly (Hoffmann et al., 2009). Also in this study, the majority of the physicians interviewed (69%) thought that the rebate policy is negatively impacting patient compliance (Hoffmann et al., 2009).
4.2.2 Impact on drug expenditure
In 2007, rebate contracts saved health insurers 310 million Euros (Rücker, 2008). However, this represents only about 1.1% of the total expenditure on drugs (Hoffmann et al., 2009).
4.2.3 Other issues
Other issues around rebate contracts include the lack of price transparency, the resulting administrative expenses, the near-total loss of cost consciousness among SHI physicians, the limited cost-efficiency perspective which only takes into account prices, the effect on reference pricing and legal uncertainties (Hoffmann et al., 2009).
In terms of competitive advantages, rebate contracts are suggested to disproportionately favour international manufacturers with broad portfolios entering the German market in comparison to medium and small size firms (Natz, 2008).
4.3. Evidence on the impact of the preference policy in the Netherlands
4.3.1 Impact on drug expenditure
The introduction of individual preference policies by several health insurers (Menzis, UVIT, CZ and Agis) in the Netherlands caused many generic products' prices to drop as much as 90% in 2008 (Foundation for Pharmaceutical Statistics, 2010). In 2009, wider implementation of the preference policy together with the introduction of the concealed price model by UVIT, ensured a
further 9% drop in medicines prices in 2009 (Foundation for Pharmaceutical Statistics, 2010).
4.3.2 Supply issues
According to the Dutch Pharmacy Association, the preferred supplier of the drug pravastatin and simvastatin was not able to ensure regular market supply for four weeks (Natz, 2008). Further, a generic producer of has withdrawn a number of molecules from the Dutch market (enapril, fluoxetine, simvastatin, tamsulosin because of price pressure from tendering (Natz, 2008).
4.4 Evidence from Belgium
4.4.1 Impact on drug expenditure
Tendering for the generic statin simvastatin in Belgium enabled a decrease in expenditure on this drug by 30% from €47 million in 2007 to €32.5 million in 2008 (VanHaeren et al., 2009). However, at the same time, expenditure on on-patent statins grew from €85.5 million to €99 million for atorvastatin and from €39 million to €42 million for rosuvastatin between 2007 and 2008 (VanHaeren et al., 2009). Overall statin market grew from €182 to €193.8 million between 2007 and 2008 corresponding to a 6.5% increase in comparison to a 10.5% expenditure increase between 2006 and 2007 (VanHaeren et al., 2009). The Belgium experience with simvastatin suggests that tendering can generate savings on a particular drug but in order for these savings to impact the overall expenditure on drugs, the shift towards more expensive medicines needs to be controlled (VanHaeren et al., 2009).
4.5. Evidence from New Zealand
4.5.1 Impact on drug expenditure
New Zealand started using tenders in 1997 and is currently tendering nearly half (by volume) of all subsidised medicines which corresponds to 20 percent of all expenditure on medicines purchased by PHARMAC (Pharmaceutical Management Agency 2011). According to official figures, this system generates about NZ$30 million savings (approx. €18.665 million) each year (Pharmaceutical Management Agency 2011).
4.5.2 Supply issues
However, as a consequence of single-product tendering, several problems have been reported in New Zealand such as stock-outs, poor quality, and potential deleterious consequences for patients who have to switch from one medication to another (MacKay, 2005). Further, it has been reported that companies who failed to win a tender withdrew their products from New Zealand's tender process and in some instances completely retired from the country's market
One of the most discussed cases of supply issues in New Zealand was the 2005 shortage of flu vaccine. Shortly before the vaccination season began it was discovered that the vaccine stock did not contain sufficient active ingredient, prompting PHARMAC to look for an alternative supplier (Blackmore, 2005). Having to find alternative suppliers for its entire market, the country ended up with an oversupply of vaccines (Blackmore, 2005). This situation raised questions about whether the risk of shortages due the single-supplier system, the related expenses and loss of public credibility are worth the savings this system generates (Blackmore, 2005).
4.6. Drug shortages in the US
The US has been experiencing a series of drug shortages in recent years.
Information on the burden of drug shortages is incomplete, however, evidence
from a 2010 survey by a US group purchasing organisation revealed that 89% of
the pharmacy expert respondents (N=311) experienced problems of drug
shortages that may have caused safety issue or medical error and 80% reported
that this resulted in the delay or cancellation of a patient care intervention
(Cherici, Frazier, & Feldman, 2011).
A study of the US Food and Drug Administration found that the number of notified drug shortages increased from 61 to 178 between 2005 and 2010 (US Department of Health and Human Services, 2011). Of 127 drug shortages studied between 2010 and 2011, 80% were sterile injectables, 28% oncology drugs, 13% antibiotics, and 11% electrolyte and nutrition drugs (US Department of Health and Human Services, 2011). Interestingly, several of the cancer drugs on shortage are out-of-patent drugs, including generics which have been used for years to treat childhood leukaemia and other cancers (Gatesman & Smith, 2011).
The official reasons for drug shortages include manufacturing problems, increase in market demand, supply delays, shortage of row material, etc. (US Food and Drug Administration). Rather, however, the list of drug in shortage seems to suggest that the reasons behind are economic ones. In other words, manufacturers do not find it profitable to supply a particular drug due to its low-price (Gatesman & Smith, 2011; Wilson, 2012).
The consequences of these shortages include increasing drug expenditure because patients are switched to branded drugs, medication errors due to inexperience with alternative products, increased labour costs due to managing shortages and adverse reactions for patents who do not have access to their preferred drug (Gatesman & Smith, 2011; Wilson, 2012).
5. Tendering policies and practices related to (generic) drug purchasing in
the Netherlands and Germany
5.1. The Netherlands
5.1.1 Evolution of the preference policy In the Netherlands, the system of preference-based policy started in 2005 with only 3 molecules: simvastatin, pravastatin and omeprazole. Concurrently, a covenant between the Government, health Insurers, the Pharmacists Association (KNMP), the generic industry association (Bogin) and the research-based industry association (Nefarma) was put in place. The covenant aimed to increase transparency and clarity about both the cost of running a pharmacy, as well as adjusting the dispensing fee to reflect pharmacy costs. At that this point in time the income of the pharmacist comprised a dispensing fee and an income out of discounts negotiated with pharmaceutical suppliers. The level of discount was monitored by the NZa (market master for care).
In late 2007 the Transition Agreement was concluded between all parties. This agreement was intended to run for two years, after which a free market should be made possible by 2010. In early 2008 health insurers wanted to speed up the process and when that failed they announced an extension of the preference policy to more molecules. These health insurers introduced an expanded list of products in the preference system from July 1st, 2008. The impact at the pharmacy level was significant, reducing the discounts accrued at a pharmacy level tremendously and, through this, achieving savings of well above €300 million (on a 12 month basis) for the insurers. The reduced pharmacy level income was theoretically compensated in 2009 by an increase of the dispensing fee costing in excess of €225 million. Since all insurers use the taxe price list (the recommended pharmacy purchase prices), the effects of different approaches to reimbursement clearly influence across insurer groups.
5.1.2 The preference policy in context All health insurers do claim that the preference system is only a means to an end and not a basic operating principle. Indeed, in order to optimize spending on off-patent medicines, many health insurance companies have developed alternative systems, intending to differentiate themselves from the rest. As a result, there is a multiplicity of schemes operating in the Netherlands in the area of generic medicines and genericised molecules, other than the preference-based policy. Starting with the taxe price, other practices or policies that are in operation include the Couvert system and the IDEA model (by Achmea and Aegis). Therefore, there are insurers where the preference system dominates, others where the preference system is not used but reimbursement takes place based
on the lowest price (which relies on the taxe), and other insurers applying different schemes. The Couvert system and the IDEA model are explored below.
(a) The Uvit Couvert system
Uvit is an insurance company that emerged from the merger of 4 insurance companies (Unive, VGZ, IZA and Trias) insures 4.2 million lives and introduced the Couvert system in 2010. Under this system, manufacturers are invited to offer discount prices directly to the insurer, using the taxe price as reference. The taxe price does not change but the selected company for a given preferred product has to pay the discount versus the taxe price directly to Uvit based on a calculation by Uvit of the number of packages sold by the pharmacist to their members.
In case of a discrepancy between the manufacturer and Uvit the former still have to pay and settle the dispute. Stakeholder perceptions differ significantly regarding the prevalence and severity of these disputes, ranging from ‘few and far between' to a ‘real problem'. In addition to the above, the contract includes a clause with a penalty to be paid by the manufacturer if they cannot supply the product for Uvit patients. The Couvert system is criticised by most parties for the unilateral (on behalf of the insurer) rules and the lack of transparency.
(b) The IDEA model of Achmea
Achmea (and now also Aegis) have introduced the IDEA model. This model is based on (a) a fixed price to pharmacy for all off-patent molecules across indications or diagnoses, (b) an increase in the dispensing fee and (c) no clawback rule. The price offered to pharmacies is based on 90% substitution and is calculated by the insurance company. In 2011, the price offered by the insurer was €2.45. According to Achmea, by mid-2012 up to 80% of the pharmacies operating in the Netherlands had joined the system. Participation in the scheme is voluntary for pharmacies and if pharmacies want to be excluded a normal preference list of products is used.
One of the operational features of the model, is that pharmacists have the incentive to search for the best possible price of a medicinal alternative in a given diagnosis and retain the difference, should that be lower than the maximum offered by the insurer, which is €2.45. Under the terms of the scheme, it does not exclude in principle any manufacturers from the market and the "winner" in this case is the manufacturer who is able to provide the largest discount to pharmacy.
CZ, another insurance company, has a comparable system in place with a pharmacy chain.
5.2. Germany
5.2.1 Developments up to 2009
In Germany, the system of rebate contracts has evolved over time, since 2004/05. As of 2008, there were four different types of rebate contracts in place.
The first type of contract was non-exclusive portfolio-rebate contracts that were concluded through negotiations and were not offered to tender. Under the terms of these contracts, Social Health Insurance (SHI) would conclude rebate contracts with every company that offered a rebate on its (entire) portfolio.
The second type of contract was exclusive portfolio-rebate contracts, concluded through negotiations and which were not offered to tender. In this case, SHI concluded rebate contracts with a limited number of (well established) companies for their entire portfolio. The decision criteria on which to award a contract were the level of the rebate and breadth of the manufacturer's portfolio.
The third type was non-exclusive rebate contract on molecule base, concluded through negotiations. Finally, the fourth type of contract was tendering on molecule basis, but not according to the provisions of the EU-procurement law. AOK started tendering on molecule base, with the key decision criterion being price (or the level of rebate).
5.2.2 Developments after 2009
Since 2009/2010 there has been a speedy evolution towards public procurement through tendering in line with the EU procurement law. This evolution has centered around two different molecule-based tender variations, whilst, at the same time, there has been a new development which centers around the creation of purchasing power among smaller social health insurance funds (SHIs). All products under rebate contract (tender or negotiated rebate contract) are marked as "preferred" products and have to be dispensed/substituted at the pharmacy. The models/variations that have emerged since 2009 are outlined below.
(a) The AOK model
The first variation relates to the market occupied by AOK, which has a 41% overall market share. According to this, and under the leadership of the AOK Baden Württemberg, Germany is divided in (8) regional lots and only 1 provider per region per molecule wins the contract.
(b) The rest of the larger SHI funds
The second variation relates to the rest of the larger SHI funds (e.g. Techniker Krankenkasse – TKK with a 7.6% market share, DAK with a 9.7% market share, and Vereinigte IKK with a 3.8% market share). According to this variation, Germany is not divided into regional lots and, in general, 3 providers per molecule are accepted, while the pharmacist can decide which product of the 3
available options to dispense. The aim under this variation is to increase the numbers of providers in order to enable greater supply reliability and more options and choice for pharmacists and patients alike.
(c) The SHI fund service companies
A third development in this context is the foundation of the new "SHI fund service companies", such as SpektrumK or GWQ+. These companies were founded out of structural changes in the SHI environment. Their aim is to provide services for smaller SHI funds, which have no considerable market shares and which, in general, have no sufficient internal structures to undertake tenders and purchase management, among others, on their own. These service companies bundle the purchasing power of up to 80 smaller SHI's, which, put together have an 8% national market share.
The remit of the service companies is to undertake all administrative tasks around the tender contracts. The terms of business usually make no use of regional divisions, three service providers per molecule will be accepted and pharmacists can decide which of these suppliers' product to dispense.
The aim was to increase the numbers of providers to get greater supply reliability, greater convenience for pharmacists and choice for patients. Of course this scheme is also associated with a series of shortcomings, including the risk of potential write-offs due to stock building, which are explored in the next section.
(d) Other variations and exceptions
There are still non-exclusive portfolio contracts (negotiated) in place for a number of molecules that are not tendered. As soon as a certain molecule is tendered, it is not object of the portfolio-contract anymore. Although not all SHI funds have moved to tender contracts, there does seem to be a trend in this direction. For example, the largest single SHI fund in Germany "BARMER GEK" with a 12.7% market share held a large number of negotiated rebate contracts up until 2011. BARMER GEK moved to a AOK tender model with single provider per molecule and no regional lots, with the first molecules being tendered in October of that year. Before this, BARMER GEK faced pressure from the BVA (Federal Insurance Agency) to move to a tender system.
6. Evidence of impact assessment and stakeholder analysis
In an attempt to assess the impact to date of the preference policy in the Netherlands and the rebate contracts in Germany, the following parameters are being discussed in the sections below: (a) impact on prices, volumes and savings from these policies to health insurers; (b) impact on the kind of competition that takes place and the barriers to entry that may exist; (c) the impact of longer time periods and patent expiry; (d) the evidence on and the likely impact of supply problems (shortages); (e) biosimilars; (f) legal aspects; and (g) impact on other stakeholders, in particular, physicians, pharmacists and patients.
6.1. Prices and volumes of products and savings from tenders
6.1.1 The Netherlands
Table 1 summarises the evidence on price competition following the implementation of the preference policy in mid-2008. These developments illustrate the fierce price competition that takes place. Over time and with successive waves of tenders, prices of widely used generic medicines have collapsed compared with the pre-preference policy period (up to May 2008), despite fears to the contrary. The introduction of the preference policy in mid-2008 had an immediate impact on prices of tendered pharmaceuticals, resulting in close to 85% reduction compared with the pre-preference policy price. Prices continued to decline and for the products shown on Table 1, price declines are averaging 94% compared with the pre-preference policy price. From the sample presented in this section there is no indication that prices are edging up. Of course, this is a limited sample with all the caveats this may imply.
6.1.2 Germany
There is no publication of rebates or winning prices in Germany. Yet, it appears that rebate contracts are becoming a preferred way of procuring generic medicines in the German context. The total generics market accounted for 22% of the German pharmaceutical market by value (or Euro 4.2 billion) and 69% by volume (26.3 billion in DDDs) in 2010. In December 2010, rebate contracts covered 56% (Euro 2.39 billion) of the total generics and biosimilars market. The share of the potential generics and biosimilars market covered by rebate contracts rose to 61% in April 2011 (IMS Contract Monitor, reported by Pro Generika).4
4 Off-patent molecules are not the only ones subjected to rebate contracts. In addition, to the figures reported in tis section, a further Euro 874 million related to in-patent medicines in 2010,
the most important of which included Rebif (16% of the total), Enbrel (15%), Betaferon (11%), Lantus (7%), and Novorapid (6%).
There has been a significant downward pressure on prices, which has driven them down to marginal cost and, as result, has compressed margins dramatically. Although price information is not available and in fact may vary across sickness funds for the same molecule, it is widely believed that significant discounts in excess of 90% off the molecule price at patent expiry are achieved.
In some cases, (e.g. the case of simvastatin), the implementation of a rebate contract is very high, such that 80-85% of total volume in a 3-slot tender and 60-65% in a single slot tender will be absorbed by the tender winner(s).
Savings from rebates have been estimated to be in the region of Euro 1 billion and might increase further if the totality of the generics market is covered. Although these savings cannot be repeated on an annual basis, since prices have already been driven down close to marginal cost, their sustainability over time implies that similar prices are achieved over successive waves of rebate contracts.
6.1.3 Stakeholder views from the Netherlands and Germany Manufacturers from both countries contend that the market covered by the preference policy or the rebate contracts is not a profitable business but allows manufacturers to stay on the market, cover their fixed costs and build image. In both countries there is wide acceptance of the fact that prices have been pushed down to marginal cost.
In Germany, despite the high implementation of the rebate contracts, the remainder of the volume is part of the aut idem (namely doctors ticking the box based on medical need to give the patient another product), which allows some diversification on the market.
In the Netherlands, the fact that the market is diversified by insurer and, as a result, the preference policy co-exists with other policies (Couvert system and IDEA model), provides flexibility and opportunity for (price) differentiation for manufacturers.
However, given the dynamics in the tender markets in both countries, it is widely speculated that over the long-term, only the vertically integrated structures will be able to stay on the market, assuming they are willing to place offers when an invitation for a tender appears.
Impact of the preference policy on prices of generic drugs in the Netherlands, 2008-2012
June 2008
Sept. 2011
Feb. 2012
May to June 2008
May 2008 to Sept 2011 May 2008 to Feb. 2012
1. Omeprazole tablets/capsules, 20mg
-88%
-94%
-94%
2. Alendroninezuur tables, 70mg
-93%
-99%
-99%
3. Omeprazole tablets/capsules, 40mg
-86%
-94%
-95%
4. Paroxetine tablets, 20mg
-82%
-92%
-92%
5. Simvastatin tablets, 40mg
-84%
-93%
-96%
6. Pravastatin tablets, 40mg
-76%
-91%
-94%
7. Simvastatin tablets, 20mg
-85%
-94%
-94%
8. Tamsulosine tablets/capsules, 0.4mg
-80%
-91%
-91%
9. Amlodipine tablets, 5mg
-85%
-95%
-95%
10. Citalopram tablets, 20mg
-88%
-94%
-94%
Sources: Stichting Farmaceutische Kengetallen (SFK); and, Kanavos, Seeley and Vandoros, 2009.
6.2. Competition, barriers to entry and (likely) impact on market structure
6.2.1 The Netherlands
Evidence on competition and market structure in the Netherlands was very patchy and did not lead to any meaningful conclusions. Withdrawals of certain manufacturers from the market had been reported, but this may have been due to other confounding factors, such as industry activity and M&As, among others. Manufacturers believe that the environment has become extremely competitive and has created a market where the length of the tender period (on average 18 months – 2 years) is potentially problematic and runs an increased risk of product write-off for those who are unsuccessful in winning preference status. In addition, competition in molecules whose patent has expired recently may be hampered if tender policies start immediately after the expiry of the originator patent, excluding all other potential providers for the length of the contracted period.
6.2.2 Germany
Market structure and competition
Several waves of tenders have taken place to date in Germany. Taking the AOK as an example, there have been six waves of drug discount contracts since 2007; the sixth wave involved in excess of 80 off-patent active ingredients with a gross turnover of Euro2.1 billion. As highlighted in the previous section, the impact on prices is largely unknown but is perceived to be very favourable to health insurers.
With regards to the market occupied by AOK, previous experience suggests that, in general, one manufacturer will win all or the vast majority of regional lots (Pro Generika, 2011, stakeholder interview; IGES, 2011). This is also a pattern that holds across health insurers, including AOK. Table 2 shows that there is indeed a pattern developing, whereby (preferred) manufacturers seem to be concentrating on particular molecules and covering the entire market. Additionally, large manufacturers, who have backward link with contract manufacturers are able to cover large parts of the market across molecules.
Germany: Rebate contract awards by insurer, 2010
Preferred Preferred
Preferred Preferred Preferred Preferred Preferred Preferred
Novartis Ratiopharm
Novartis Winthrop
Note: 1
Insurers other than AOK have the ability to award contracts to three providers.
Source: Pro Generika, stakeholder interview, 2011.
Dividing the market in regional lots was supposed to increase the number of participating providers/companies and to strengthen medium-sized businesses, but this appears not to have worked and we see instead increased concentration in the AOK tender results (Table 3). In a recent study by IGES (2011), the top-10 seller concentration ratio was 49.2% across the entire generics market, but registered 75.4% in the rebate market and was only 35.3% in the non-rebate market. In successive waves of AOK tenders, the top-10 firm concentration ratio increased from 91.4% in the third wave (June 2009-May 2011), to 93.7% in the 4th wave (April 2010 – March 2012) and to 97% in the 6th wave (June 2011 – May 2013). It appears that rebate contracts favour larger firms and it is unclear how this is affecting smaller players on the market, although it looks as though smaller players are in principle less able to take advantage of the rebate market.
Market share and concentration levels in successive AOK tenders, 2009 - 2013
3rd wave of AOK tender
6th wave of AOK tender
4th wave of AOK tender1
(1 June 2009–31 May 2011)
(1June 2011-31 May 2013)
(1 April 2010-31 March 2012)
Turnover: Euro2,375,257,157
Turnover: Euro2,097,522,771
Turnover: Euro1,516,430,977
Torrent (Heumann)
Top-5 concentration ratio:
Top-5 concentration ratio:
Top-5 concentration ratio:
Torrent (heumann)
Top-10 concentration ratio:
Top-10 concentration ratio:
Note: 1 The molecules under this wave are different to the ones under waves 3 and 6.
Source: Adapted from IGES, 2011.
Rebated originator and generics
A further issue related to competition is the possibility to establish contracts with patented products a few months prior to patent expiry. The case of Cellcept is quite interesting in this regard. The contract is still valid after patent expiry and Cellcept is marked as a preferred rebated product in the pharmacy-software and has to be dispensed preferably. Although there are rebate contracts for the originator as well as for 6 generic products, the physician prescribes and the
pharmacy dispenses the originator. Seven months following the patent expiry of the originator (Cellcept), the market share of the rebated generics was in the region of 4.2% in total (Figure 1). The list price for Cellcept has not decreased, but the rebate given to health insurance is unknown and it may well be the case that the amount of rebate offered matches that of the generic competitors, but no information is available to validate this claim.
Germany: Development of generic market share for Cellcept, 7 months following
is patent expiry (November 2010 – May 2011)
Source: Insight Health 2011, personal communication.
Another similar example is that of Olanzapin, Zyprexa, where the market share
by volume was retained for roughly five months in a region using rebate
contracts, in spite of coming off patent. This contrasted with swift entry of
generics in regions that were not using rebate contracts for Zyprexa when it
came off patent, as is illustrated in Addendum 1 (Pro Generika, 2012). Again, this
is only problematic for insurers if the price does not reduce to be comparable
with the generic entrants, information we do not have access to.
Impact of rebates on the business model and market structure
In order for manufacturers to respond to the competition that results in markups that are virtually nil, they have had to change their business model in a number of ways, which, in turn has begun to affect market structure.
Some of the larger manufacturers do not make tendering offers because they are a strongly recognised brand, but leave this to specifically established entities (spin-offs or independent "subsidiaries"), which have been created with the sole purpose of leveraging the tender market. These entities have no sales force and incur no marketing expenses.
Other manufacturers, particularly, those who are of average size have had to adapt to the new environment of rebate contracts and have abolished their sales forces and/or downsized their headquarters. For these manufacturers, the rebate contract business has resulted in a dramatic cost-cutting exercise. In the majority of cases, a team of people is sufficient (in one interview it was mentioned that a team of 10-12 people would be adequate for this purpose) to put offers together. For these manufacturers diversification into niche markets remains a key objective.
In light of the continued downward pressure on prices due to the rebate contracts, manufacturers are identifying niches and diversifying their portfolio of activities as a means of staying on the market. Sources of diversification include specialty areas, such as CNS products or women's health products, OTC medicines, and biosimilars.
6.2.3 Stakeholder views from the Netherlands and Germany
In light of cost pressures, manufacturers that currently produce in Germany are highly likely to (further) downsize or altogether shift any remaining manufacturing activity elsewhere spreading it across their affiliate population or by sub-contracting.
While it appears that many manufacturers rely on sub-contractors (located both in Europe and elsewhere), it was felt that it is only a question of time for a significant proportion of these sub-contractors to feel the pressure of low and declining prices. This could mean that production may be shifted further afield.
In a few cases it was mentioned that manufacturing of a particular molecule would concentrate in one site globally, which, given the price pressures could provide economies of scale (EoS) in a low-cost environment. In this case, manufacturing would probably need to relocate outside Europe, resulting in a scenario where a significant proportion – if not all – of a particular product will come from outside the EU.
Given the peculiarities and price pressures in the tender markets, a number of manufacturers highlighted the possibility of altogether exiting this market in the future and concentrating on other markets, both in Europe and third countries. It is highly likely that companies will disappear from the German market in future. Current developments point to generic firms operating without medical representatives.
Finally, nearly every interviewee highlighted the need to diversify in areas where tenders are not so common (e.g. respiratory products; paediatric medicines); niche markets (e.g. women's health), biosimilars, special therapeutic areas and institutional business, such as home care (for specialised products).
6.3. Impact of longer tender periods and patent expiry
Two further issues have been identified, which in their own right may have an impact on the type of competition that takes place in an environment characterised by tenders; these relate to the (a) actual tender period and whether this is long and (b) the time lag for the issue of a tender following the expiry of the patent of a molecule. Both issues have been raised in discussions with stakeholders in the Netherlands and Germany.
6.3.1 Long tender periods
In Germany and from a legal standpoint, the length of time between tenders is 2 years, while in the Netherlands this ranges between 1.5 and 2 years. This is often considered to be too long a period for unsuccessful competitors to stay on the market. Insurers, on the other hand, argue that with shorter contract periods the tender market develops into a spot market.
Longer tender periods may decrease manufacturers' flexibility of supply on the market and companies will reduce their stock capacity in order to avoid financial risk. This leaves very limited room for addressing any eventual shortages in the market. Other manufacturers may simply exit the market if they do not manage to win a tender, as the slot for a particular product will not be available for a long time period.
As a result of these suppliers exiting the market, longer tender periods may have the indirect effect of supply problems and pharmacists have claimed that the search costs of medicines procurement and the administrative aspects have increased considerably.
6.3.2 Tenders issued immediately after the expiry of a patent
An additional issue arises with regards to the point in time when a tender is issued after the expiry of the patent of the originator product. It is understandable that health insurers believe their best interests are served by issuing a tender immediately after the expiry of the patent of the originator product. Evidence from Germany suggests that they do so – at least with regards to molecules that are significant from a commercial perspective. For the molecule in question, the tender was issued the month following the expiry of the patent for the molecule in question (Table 4).
The challenge from a generic supplier's perspective in this context is twofold: first, suppliers entering the market late cannot participate in the tendering process and are altogether excluded from the market for this product. Second, if all insurers issue tenders immediately after the expiry of the patent of the originator molecule, then there is practically no market potential for newly genericised molecules outside the tender market. Both these aspects may have an impact on the amplitude and extent of competition that takes place following genericisation.
Germany: Relationship between Patent expiry and tenders issued, 2009-10
Source: Data acquired via Pro Generika.
6.4. Evidence on supply problems
Despite expectations to the contrary, supply problems seem to occur more regularly than not in the Dutch market, although evidence on shortages in Germany is scarce. A recent list (May 2011) provided information on shortages of 80 preferred products (30 molecules) in the Netherlands. Stakeholders suggest that supply challenges are present throughout the contracted preference period. A new monitoring system based on national wholesaler data has reported that in an average week 120 of 180 preferred products face availability problems (Pharmaceutische Weekblad, 2012).
Problems with the supply of preferred medicines may be due to a number of interlinked reasons. Supply gaps at the beginning of preference or rebate periods may be attributed to inadequate lead-times between signing the contract and beginning supply (lead time). Manufacturers claim they require around six months to engage production capacity for the awarded volumes and the two to four month period available to them is inadequate. In an attempt to avoid delayed supply capacity providers stock-build in advance without knowledge of
whether they will be awarded a contract. This creates an additional risk for small and medium sized companies due to high write-off potential, ultimately reducing competition and suppliers as smaller providers exit the market.
Supply challenges throughout the rebate or preference period may also be a result of suppliers' inability to meet required volumes because of capacity constraints or miscalculations (Stakeholder responses, 2012).
Supply defaults from awarded providers lead to contractual penalties, high transaction costs for pharmacists, who are legally obliged to hunt down and provide the preferred product, replacement purchase costs that are difficult to calculate, claims for compensation, as well as to a single sided right of cancellation of the contract for the SHI Funds
6.5. Biosimilars
6.5.1 Current and future market potential
Biosimilar product approvals are based on stringent submission requirement, including substantial clinical studies. From a European perspective, the package of data and information required to bring biosimilar drugs to market include quality data, 5 non-clinical data, 6 clinical data, 7 pharmacovigilance, 8 and assessment of immunogenicity, which is submitted alongside the clinical data. As a novel segment, biosimilar medicines are proving a challenge to bring to market. This is also shown on Table 5, which outlines the 4 original biotechnological substances and the biosimilars that have emerged to date.
5 The quality of the biosimilar must meet the same requirements and standards as that of the
reference product, namely manufacturing process, consistency of manufacturing, analytical tests, and stability of the product. 6 The registration dossier for biosimilar medicinal products includes comparative non-clinical data and the requirements are usually product-specific, but usually include: a short-term
toxicological reeated dose study, pharmacokinetic/pharmacodynamics studies in an appropriate animal model and local tolerance testing. 7 For instance, a summary of the results of clinical trials conducted in patients and healthy
volunteers with a biosimilar product. For most biosimilar products extensive comparative trials have been conducted, often involving several hundreds of patients. 8 Through the submission of a risk management plan –RMP- which is detailed description of the
manufacturer's risk management system.
Overview of currently licensed biosimilar products in Europe, 2011
MA Holder
Date of MA
Tradename
Reference
Sandoz GmbH 12 Apr 2006
Sandoz GmbH 28 Aug 2007
Epoetin alfa Erypo/Eprex Hexal
Teva Generics 15 Sep 2008
Source: EGA Biosimilar-Handbook, 2011, Pro Generika communication.
In addition to the currently available biosimilars on the market, prospective patent expiries raise significantly the potential for biosimilars, particularly in the field of monoclonal antibodies. The combined sales in Europe and the US of biopharmaceuticals with high biosimilar market potential, such as Enbrel, Remicade, Avastin, Humira, Lovenox, Mabthera and Lantus, exceeded US$30 billion in 2009. Penetration by biosimilars after the expiry of the patent on the above products would imply a price reduction by a certain proportion, which would be beneficial to health insurers.
6.5.2 Evidence on tendering and stakeholder perspectives
The situation on tendering for biosimilars is quite complex and difficult. There is enormous opposition from the originator companies claiming that biosimilars are not exchangeable (substitutable) with the original products. Evaluation on the basis of EMA criteria of submitted dossiers suggests that biosimilar products are considered to be comparable from a clinical and safety point of view. The policy view, however, is that no (generic) substitution should take place, but that the physician needs to be involved in treatment decisions concerning substitutability.
In the Netherlands, there is currently no preference policy for biosimilars, although this is at this moment under heavy discussion. Some insurers wanted to start tenders for biosimilars; but the view at the time of writing was for preference policy to apply to new patients only. As meetings with stakeholders suggested, the dynamics around this view could be quite compelling; for example, new patients for human recombinant growth hormone (HRGH) are 4% and if one manufacturer wins the tender for that market, then there is the other 96% of the market to fill. Additionally, some products (TNF-alfa inhibitors) have been shifted into the hospital from the retail segment, with a reduction in overall spend.
6.6. Legal issues
Tender practices have been the test bed for legal challenges over the past few
years, particularly in Germany (Kanavos, Seeley, & Vandoros, 2010). This is also
reflected in the number of companies taking legal action as well as the number of
lawsuits heard over time (see Table 6). Due to new legislation sickness funds
have to subscribe to § 97 (3) of the German Act against Restraints to
Competition (GWB). According to the provisions of this legislation, public
procurement needs to take into consideration the interests of medium-sized
businesses (Mittelstand); additionally, partial lots and regional lots have to be
tendered and the law places limits on the procurement bundling power of
sickness funds. Finally, it is now a requirement for the duration of rebate
contracts to be 2 years.
Despite the above, legal challenges continue to take place. This essentially highlights the fact that the legal ramifications in this part of the system may still need to be further clarified, despite the clarity surrounding the EU procurement directive. One such issue has arisen in Germany, where the right to award the tender by a sickness fund (BKK) to three providers has been challenged on the grounds that two of the "preferred" manufacturers have large sales forces which can be perceived as unfair competition.
A legal dispute between GSK and Teva in the Netherlands provides another example relevant to competition concerns for the generics market. Generic suppliers cannot submit a price to the taxe prior to the expiry of patented products since this is considered a commercial act. This leads to delays of on average six weeks before generic products enter the market, decreasing the chance of being selected as a preferred product for the subsequent period, as well as increasing the risk of litigation in a low-profit margin environment. Although this does not affect the preference policy per se, it becomes less attractive for generic companies to introduce new products, potentially reducing competition.
Legal disputes for AOK Tenders, waves I – VI; 2009 - 2013
companies taking legal action Number of
lawsuits
Source: AOK Baden-Württemberg, 2011.
6.7. Implications for other stakeholders
6.7.1 The Netherlands
From a Dutch perspective the greatest opposition to the preference policy, other than manufacturers, comes from pharmacists, who have seen their discount levels (and thus income) dissipate as a result of the policy. Amendments have been made to the level of the dispensing fee in order to enable pharmacists replace the lost income from discounts and this has in part annulled the original savings from the preference policy. A new remuneration system for the pharmacist was introduced in 2012 but pharmacy stakeholders perceive this as bearing no significant departure from the previous system. Based on 11 pharmaceutical care parameters pharmacists and health insurers have to agree on a fee for pharmaceutical care.
A further aspect of contention among pharmacists relates to stocking issues and the increased transaction costs they face as part of the preference policy. If the pharmacy contracts with numerous insurers it is obliged to supply every
preferred product, or risk litigation. The logistical services fees are faced solely by the pharmacist, who is obliged to provide contracted patients with the relevant products, regardless of the volume of patients.
Major reactions from doctors and patients have not been recorded, although doctors have to explain differences in treatment therapy to patients as a result of the preference policy. Patients often need to be offered explanations about likely changes to their medications and their appearance (colour, size, shape, etc). All the above might have an impact on patient adherence to treatment, although hard data to support this is not available.
6.7.2 Germany
From a German perspective, it appears that oppositions to the rebate contracts
are less significant than in the case of the Netherlands. The landscape for
stakeholders other than manufacturers and sickness funds between 2009 and
2011 has not changed. For physicians, rebated products are taken into account
in line with the assessment of cost-effective prescription habits of the physician.
Physicians also have to explain to patients any changes to the product the latter
are receiving, which can be time consuming, although in recent years, it appears
that patients are on aggregate informed about the rebate contract provisions. For
pharmacists, there are no comprehensive direct financial incentives for
pharmacists; only in one case, AOK reimburses the dispensing of rebated
products with an additional 1 Euro per script. Transaction costs and stocking
issues are also points of contention.
Products under rebate contract can still have patient co-payment, even if there
are similar products in the market that are free of co-payment. From a German
perspective, it appears that the acceptance of rebated drugs is quite high based
on a survey of AOK insures conducted in late 2009, as shown in Figure 2. Patients
seem to accept discounted drugs in large proportions on the understanding that
no differences exist in quality. They also seem to be more sensitive to paying the
co-payment than accepting the discounted drug.
Assessment of discount contracts from the point of view of AOK insures,
2009; N=2025; affirmative responses as % of total responses
Drug manufacturer does not make a
difference so long as quality is OK
I don't mind taking a drug that is
covered by some discount contract
The quality of drugs dispensed by
pharmacies is high
I don't feel disadvantaged if the drug
dispensed to me is a discounted drug
Discount drugs are just as good but
Drug manufacturer does not make a
difference as long as I am exempt…
The quality of discounted drug is just as
Source: Schroeder & Selke, WIdO, presentation delivered in Brussels, 24 November 2009.
7. Discussion
7.1. Advantages and limitations of tender practices for health insurers and
manufacturers
Like all policy measures, tenders have both advantages and disadvantages for different stakeholders. The two key stakeholders involved in this context are health insurers and manufacturers. Each of them views tenders differently and is differently affected by their implementation.
7.1.1 Health insurers
Tender practices offer several advantages to payers/health insurers. First, payers/health insurers clearly emerge as the determining players and the balance of power seems to have shifted in their favour since the implementation of tenders in the Netherlands and Germany. Second, tenders are viewed as the primary vehicle for achieving the lowest possible prices from drugs subjected to them, whilst at the same time ensuring that supply will not be adversely affected. A surrogate advantage is the ability to maximise savings and sustain these over time, on the understanding that tender prices remain low over successive cycles.
The limitations that payers/health insurers face relate, first of all, to the long-term sustainability of the prices achieved in previous tender cycles. There is always a fear that changing market dynamics (partly because of the continued pressure on prices and the subsequent exit of some providers), might lead to price increases, although this remains hypothetical. A further limitation relates to the size of the savings and the level of acceptability by other stakeholders. In the case of the Netherlands, health insurers need to engage with pharmacists to ensure facility level supply challenges are resolved. Clearly, the changes to pharmacy reimbursement implemented in NL were necessary, and the impact they have should be monitored. As the German experience suggests, positive (financial) incentives might also be considered, although the practice remains limited. A third limitation relates to legal challenges by providers, who feel that the existing framework does not safeguard their interests. A final challenge relates to the operational robustness of the tender system. Although all manufacturers are required to submit proof of capacity to supply, this is occasionally not followed, potentially resulting in supply shortages during the contract period
7.1.2 (Generic) Manufacturers
For manufacturers, the limitations of operating under a tender system exceed the advantages. The main advantage for a provider is the guarantee of the totality (or a substantial proportion) of the market if a tender is won. Limitations include the significant uncertainty about the extent to which a tender can be won, the race towards the bottom in an environment where prices among
competitors are not known, the exclusion from the market if a tender is not won, which is linked to the length of the tender period and the sunset clause if a manufacturer stays too long out of a product market. Additionally, tenders do seem to have an impact on different providers' business models, leading to employment reductions, and can have an impact on industry market structure, levels of concentration and ability to compete.
Providers' response to the above would be to either exit the market, opt out of the tender systems, or adapt and diversify; with regards to the former, there is little evidence to suggest that tenders have induced providers to exit the production of generics, although this could be because this practice is not yet widely implemented across Europe to induce such a dramatic change. What seems to be happening, however, is that the seller concentration is increasing over time and across several waves of tenders. On the other hand, there is sufficient evidence that providers do adapt and diversify their business accordingly by expanding into areas and segments not directly affected by tenders or becoming more active in specialist markets.
7.2. Challenges of the current tendering models in the Netherlands and
Germany
7.2.1 Lessons from the two countries A number of challenges characterise the implementation of the tenders/rebate contracts in the Dutch and German contexts.
From a German perspective, there are significant challenges, although health insurers are content with the level of expenditure and the savings achieved and also argue that – on the basis of surveys - patients are not dissatisfied with how the system operates if there is a financial benefit to themselves of their health insurance funds. The first challenge is that there is no publicly available information about winning prices, as these are commercial secrets and are part of the ownership of SHI funds. To that end, it cannot be ascertained what the level of discount is due to the lack of transparency.
For manufacturers the main challenge beyond reduced margins is the lack of certainty about market penetration and uptake. Under the AOK tender variation, which essentially allows only one manufacturer to win the contract in each lot, it is unclear, what the implementation rate will be for successful providers, due to the behaviour of prescribing physicians and the aut idem system.
For those outside of the system, awards within the AOK tender variation imply that, as one interviewee put it, the preferred product is in "driver's seat", meaning that it is likely to be delivered more widely to all patients in general. This is because with AOK's 41% nationwide market share that product will be on
stock in nearly every German pharmacy and pharmacists are often not willing to hold a second provider in stock, due to storage space restrictions.
Even under the rest of the larger funds' variation (enabling up to 3 providers to win contracts at national level), there is still high uncertainty for successful providers, as it is still unclear which option the pharmacist will dispense. For instance, if one provider out of the three is well established with additional significant capacity to discount to pharmacy, or able to market to physicians more aggressively, the pharmacist may dispense this product instead, therefore well-established providers may obtain a (much) higher market share than the (remaining) two less well-known providers.
A challenge for both insurers and providers relates to changes in competition. It appears that instead of intensifying competition, tendering has led to increased seller concentration, particularly in that part of the system that is characterised by a single provider (the AOK variation) and that provider size is in the majority of cases a determining factor. Thus, it can be argued that tenders act as entry barriers to smaller firms. However, it is not known what impact this increase in concentration levels has had on the winning prices over time, making it impossible to comment on the extent to which price competition has been affected.
Another competition-related aspect relates to the co-existence of rebate contracts for originators and non-exclusive portfolio rebate contracts for generics. As the cases of Cellcept and Zyprexa in Germany highlight, the market share for generics is not always optimised. From the sickness-funds' point of view, this is not necessarily bad news if the rebate of the originator is close to the rebates offered by the generics, but, if not, then it is an issue.
The Netherlands
From a Dutch perspective, the preference policy also presents challenges despite the low prices it has generated for health insurers over the past 3 years.
The first two challenges relate to how the preference policy is implemented and impact it is having on the pharmacy market. Firstly, the operational peculiarities of the pharmacy reimbursement system mean that significant financial risk is placed on the pharmacists. Historically pharmacists relied on discounts for part of their income (part of which is clawed back by insurers) and currently have to operate with a multiple set of systems involving either no discount (under the preference policy) and some discount (under the IDEA model), potentially creating confusion and high transaction costs.
The second challenge relates to the functioning of the retail market and, in particular, stock and logistics issues. Transaction costs and stocking issues can increase considerably for pharmacies since different insurers have different preferred drugs and if a pharmacy does not have a preferred drug it potentially
violates its contract with the insurance in question. Having to navigate multiple reimbursement systems for generic medicines in the Dutch health insurance market places significant pressure on pharmacists. For "preferred" drugs, the time between award and implementation of a contract is considered to be relatively short for stock movements.
Having said this, from a provider's perspective, the presence of other reimbursement systems or methods e.g. the Couvert system and the IDEA model, allows manufacturers to cross-subsidise for reduced margins or losses they face in the preference system. In this way the multiplicity of schemes can be seen as a double-edged sword.
The third challenge is the lack of availability for a number of products on the Dutch market. This has been raised on a number of occasions and for several products, despite the pledge by manufacturers to avoid these situations. The lack of availability seems to be pervasive throughout the contract period. Challenges at the start of a product's preferred contract might be partially attributable to inadequate lead-times, but during the contracted period it appears to be more related to manufacturer supply defaults or challenges in logistics between wholesaler and facility level. Manufacturers that are not preferred have an incentive to stock-out and probably exit the market. The lead times in order to re-stock are in the region of seven months and this can create an issue if preferred manufacturers face an unexpected supply problem.
7.2.2 Common practical challenges of tenders
The implementation of tenders/rebate contracts presents a number of practical challenges to all manufacturers who make tender/rebate offers. These relate to regulatory compliance, quality assurance and legal implementation.
Regulatory compliance
Regulatory compliance is intense, requires significant attention and its costs should not be under-estimated. The European procurement law is often perceived to be very formalistic and requires detailed information by those who submit offers; companies can be disqualified if they do not submit the right information to the authorities (e.g. during interviews it was mentioned that a company was disqualified because the electronic signature was not the right one). In addition, all steps into the system have to be confirmed and this relates to all parts of the supply chain to ensure that they can deliver the product(s) for the 2-year duration of the contract, even in the cases of sub-contracting.
Quality assurance
Quality assurance might be at stake if companies always need to supply at the lowest possible price. Many manufacturers are trying to shift supply across their
affiliate population, or by sub-contracting within or outside Europe, including India. Ultimately, however, the winner(s) of the contract is (are) responsible for safeguarding quality and the penalties for not doing so can be significant, in addition to any replacement costs.
Legal challenges
There are also legal challenges, which essentially highlight the fact that the legal ramifications in this part of the system may still need to be further clarified, despite the clarity surrounding the EU procurement directive. One such issue has arisen in Germany, where the right to award the tender by a sickness fund (BKK) to three providers has been challenged on the grounds that two of the "preferred" manufacturers have large sales forces which can be perceived as unfair competition.
Another example is the dispute between GSK and Teva in the Netherlands discussed earlier. This illustrates the need for increased understanding of how generic firms navigate the risk of litigation with the need to access the market quickly in order to participate in reimbursement processes. The entry of generics on the market can be delayed by several weeks because submitting a price to the taxe prior to the expiry of the patent is considered to be a commercial act. Although this does not affect the preference policy per se, it does mean that generics might be excluded from tender processes because of having delayed market entry.
Longer tender periods
Longer tender periods may lead to long-term supply problems, since non-contracted suppliers will exit the market, making spot purchases difficult in the event of supply defaults. Additionally, as potential suppliers dwindle the system becomes less flexible and faces reduced competition, potentially leading to increased prices in the longer term. Secondly, the potential discontinuity of a product that has lost its preferred status, particularly if the company faces stock-write offs because they cannot shift the product to another market segment.
Lack of availability
Problems with the supply of preferred medicines may be due to a number of interlinked reasons. Supply gaps at the beginning of preference or rebate periods may be attributed to inadequate lead-times between signing the contract and beginning supply (lead time). Manufacturers claim they require around six months to engage production capacity for the awarded volumes and the two to four month period available to them is inadequate to make sufficient provisions in order to supply the entire market.
Supply challenges during the rebate or preference period may also be a result of suppliers' inability to meet required volumes because of capacity constraints or miscalculations. The issue of defaulting on supply is more difficult to address for
manufacturers where production is vertically fragmented, with parts or all of production spread geographically or between multiple companies, as is the case to some extent in both the countries explored here.
Within a single-supplier environment the risk of a supplier default during the rebate contract or preference period has significant implications, since it necessitates off-contract purchasing. This challenge is augmented by the high concentration of suppliers, which means there are fewer options outside of the tendered market. The issue of defaulting on supply is also more difficult to address for manufacturers where production is vertically fragmented, with parts or all of production spread geographically or between multiple companies, as is becoming increasingly common in Europe.
Generic market access
Issuing a tender immediately after the expiry of the patent of a molecule may have significant implications for competition. The challenge from a supplier's perspective in this context is twofold: first, suppliers entering the market late cannot participate in the tendering process and are altogether excluded from the market for this product. Second, if all insurers issue tenders immediately after the expiry of the patent of the originator molecule, then there is practically no market potential for newly genericised molecules outside the tender market. Both these aspects may have an impact on the amplitude and extent of competition that takes place following genericisation. If a rebated product comes off patent during the contract period in Germany there is no renegotiation to incorporate generic molecules. Generic manufacturers criticise this practice (Stakeholder communication, 2012).
Biosimilars
The potential for biosimilars remains largely unrealised and the view is that physicians need to be involved in decisions about which product patients ought to receive. Starting a therapy on a new patient seems to be less contestable and this presents opportunities – currently – for biosimilars. The future potential for biosimilars is significant as a large number of monoclonal antibodies are expected to go off-patent. Although many argue against this case, because of substitutability concerns, there is definite scope for increased penetration by biosimilars, and this should be considered by insurers in one form or another.
Stock write-offs
The short lead-time between acceptance of the bid and start of the tender-contract leads some manufacturers to stock-build in advance, without the knowledge of winning the tender. This creates an additional risk for small and medium sized companies due to high write-off potential.
Pharmacists also face the risk of stock write-off when insurers change providers, particulalry if their stock levels have not been carefully managed, or if they cannot shift stock to other patient groups who are not on rebate or preference contracts.
7.3. Prices achieved and savings to health insurance
The prices achieved in both the Netherlands and Germany on molecules tendered are very favourable to health insurers and sickness funds. The evidence from the Netherlands suggests that since genericised molecules have been subjected to tendering, the price levels achieved are consistently low over several tender cycles compared with the price of the molecule at patent expiry. The discount off the price at patent expiry exceeds on many occasions 93% to 95%. Publicly available information on prices achieved is not available in Germany, although interviews with stakeholders suggest that they lie within the same or a comparable range (ie exceed 90% discount off the price of the molecule at patent expiry). The tender prices achieved in both countries are well below list and molecular reference prices and, as a result, it is hardly surprising that tenders have become an established and preferred means of procuring off-patent medicines across a range of health insurers or sickness funds in the two study countries. The savings achieved by procuring off-patent medicines through tenders can be significant and have been shown to run up to Euro 1 billion in Germany. Although this is a one-off saving for the relevant market segment, sustaining it implies that the same or comparable price levels are achieved over successive cycles of tenders for the same products, which, prima facie, does not seem to be a problem. It is evident that low prices at this level pose questions around sustainability over the longer term. Alongside lower prices and the resulting potential savings, tenders offer other tangible advantages to health insurers; these include low administrative costs in an environment where manufacturers need to guarantee high quality supply; they also include competition, or, rather a race towards the bottom, as manufacturers are not aware of each other's pricing strategy and are prepared to outbid each other by offering a price close to marginal cost in order to win the tender and stay on the market.
7.4. Tendering in an environment of multiple insurers
It was highlighted that tendering practices in Germany and the Netherlands are now extensively used by different sickness funds (Germany) or insurers (the Netherlands). Based on the evidence from both countries, operating in systems characterised by multiple insurers/sickness funds offers – in principle - certain
advantages to generic competitors. This occurs for two reasons: first, the market for a molecule to be tendered can be fragmented and different insurers/sickness funds may issue their own tender and at different points in time; additionally, depending on their organisational structure, tender invitations can run regionally rather than only nationally (as occurs in Germany). This potentially offers greater opportunity to generic manufacturers to compete for business at different points in time and potentially increases the odds of winning one of the tenders. Second, tenders may run in parallel with, or may be one of the components of different policies pursued by insurers (as occurs in the Netherlands, where preference-based policies co-exist with other policies or practices such as reference pricing, the Couvert system, and the IDEA model). Based on the Dutch experience, multiple policies and practices may result in an environment for competitors where the more predictable policies provide a cushion for the less predictable ones (tenders).
The above have important policy implications for countries whose health care systems are characterised by a single purchaser, for example, a single insurance fund or a tax-based health care system with a single purchaser operating at the centre, both known as a monopsony. If a tender policy is introduced around the lowest-price-wins-all principle and with a minimum duration of 1 year, then implementation of a single tender per molecule in a monopsony environment might have short-lived effects for the monopsony, as the market will be effectively closed to all other competitors, who may exit and find it difficult to re-enter. The wider implementation of tendering practices in systems characterised by monopsonistic structures, therefore, needs to be carefully thought through and organised in ways that would safeguard opportunities for as many players to compete on the market at as many points in time as is feasible.
7.5. Impact on generic market structure and future competition
Besides the low prices and savings achieved by health insurers/sickness funds, there is some evidence that the wider implementation of tenders in Germany and the Netherlands, has begun to affect the generic industry market structure. This has manifested itself in a variety of ways. Some manufacturers are no longer engaging with or participating in tenders and have downsized their operations already. Other manufacturers have changed their business models and established subsidiaries that are only dealing with tenders; this implies zero sales force in the market concerned, reliance on sub-contractors to deliver product or concentration of manufacturing on one location in order to be in a position to compete. In light of reduced profitability from the tender business, most manufacturers are seeking opportunities to diversify their business portfolio by identifying niches (e.g. women's health; special therapeutic areas which are not necessarily subjected to tenders, OTCs).
It appears that tendering in both the Netherlands and Germany is beginning to have a profound effect on manufacturing market structure, as several manufacturers, particularly in Germany, are uninterested in the tender business because it is considered to be a low margin business. From a German market perspective, this is in part understandable because it used to be a market that relied on the supply of branded rather than unbranded generics. The adjustment to a market characterised by tenders with an increasing implementation rate has been accompanied by a loss of employment over the short-term, as manufacturers have laid off a significant proportion of their sales force. Perhaps more worryingly, the tender business has already shifted production to sub-contractors either elsewhere in Europe, or, more forcefully, outside Europe. As there is constant downward pressure on prices, it has become a necessity to seek competitive partners at global level who can supply at minimum cost and acceptable quality. It is not unreasonable to think of circumstances in the future, where the majority of production will have shifted outside Europe. The more countries join the tender league, the more realistic this prospect becomes.
7.6. Industrial policy for the generics sector
The changing environment and market structure raises the question of whether there needs to be an industrial policy consideration for the European generics business. The evidence suggests that health insurers and sickness funds are not making contract decisions based on industrial policy considerations, but on cost reasons. While this is understandable, it also raises questions about the overall survival of the generics sector in Europe. This may be an undue concern as generic manufacturers are clearly taking steps by diversifying their portfolios and business models to the new reality, but it begs the question of whether a wider stakeholder reflection might need to take place in this regard.
While production is shifted to other European countries and, increasingly, outside Europe, there is some concern that the shift to third countries is creating an uneven playing field for manufacturers operating in Europe, who face additional regulations because of the environmental and social protection requirements. As such, one might well argue that these considerations should also be considered in the price.
Bearing in mind the above, national and, perhaps, EU-level decision makers may want to reflect on whether there is a need for an industrial policy for generics, whether this sector is strategic for Europe's supply of medicines and, if so, what components this industrial policy might have.
7.7. Product supply and shortages
Although tender awards are usually accompanied with guarantees on supply, there have been circumstances where manufacturers were unable to supply agreed-upon quantities. Although there is no evidence of this being a significant problem in Germany, the Netherlands appears to be facing pervasive problems with adequate supply of preferred products at the pharmacy level. A new monitoring system based on national wholesaler data has reported that in an average week 120 of 180 preferred products face availability problems (2012). Insurers need to have contingency plans in place and interrogate the causes for these supply challenges. Managing supply issues becomes difficult if the entire market is subject to the same scheme.
7.8. Impact on other stakeholders
The impact of tenders and tendering on stakeholders other than insurers/sickness funds and manufacturers can be significant and must not be under-estimated.
Patients have on certain occasions expressed concern and confusion about a medicine being changed to one that has different shape or colour. This may be of importance to elderly patients with multiple co-morbidities who are receiving multiple medications and are used to a well-established pattern where medicine recognition acts as a safeguard and might affect compliance and adherence to treatment, although hard data to support this is not available.
The solution to this problem is not necessarily more regulation (e.g. about how to supply a particular medicine and what colour it should have), but, perhaps, continued information from health insurers and greater interaction with pharmacists. This is already reflected in patient views in Germany in a survey about rebate contracts. Physicians also need to be in a position to explain changes in the product supplied to their patients and this can be time consuming.
There are differences in the reaction of pharmacists between Germany and the Netherlands. In Germany, pharmacists are at best neutral, but this is partly reflected in their payment schedule remaining unaffected and in one case incentives being offered to compensate for time to explain to patients about rebate contracts. In the Netherlands, the preference policy has altogether extracted the entire amount of discount that was in the system and which formed part of pharmacists' income. Although the dispensing fee was increased (ex-post) to reflect this loss, skepticism and suspicion has remained among the profession. In both cases, however, tender policies have increased transaction costs and the costs of procurement and stocking. The challenge of maintaining contractual obligations with multiple insurers was highlighted by pharmacy stakeholders as an insurmountable challenge, with negative financial
implications. Particularly, since many pharmacies are small medium enterprises, they are unable to negotiate procurement from multiple wholesalers, and will therefore find it impossible to adapt to supply issues. The implications of these challenges may become increasingly clear. During September 2012 MEDIQ announced that it would be restructuring Pharmacies Netherlands in response challenging reimbursement context for pharmacies and wholesalers, laying off 134 employees (MEDIQ, 2012) . Overall, important lessons can be learned from the experience of tenders to date. While the short-term economic rationale of tenders is undisputed the likely impact on other stakeholders needs to be taken into consideration and negative effects minimised.
7.9. Sustainability over the longer term
Ultimately, the question that needs to be asked in this context is whether payers/insurers, the generics industry, and other stakeholders can operate under the same terms and conditions over the longer term. In other words, will the system in its current form be sustainable in the future.
As far as payers/insurers are concerned, the strategy will always be to obtain the best possible prices for genericised products, and across groups of products that are considered by insurers to be (perfect) substitutes, on the understanding that quality and continuity of supply can be guaranteed and maintained for the contracted period. Dutch insurers (subscribing to the preference policy) and German sickness funds (aggressively pursuing rebate contracts) have shown they are not prepared to pay any premium for off-patent medicines.
As far as the generics industry is concerned, it is clear that the operating environment for most European players is becoming increasingly challenging. In order to deliver – in a sustainable manner - the prices that health insurers have got used to over the past few years, it looks as though changes in market structure and manufacturers' business model are inevitable. Tender prices for many genericised molecules achieved in the Netherlands and Germany are very close to the international prices for the same active pharmaceutical ingredients. Spare capacity that can deliver these prices can be found either through sub-contracting elsewhere in Europe, or, more realistically, outside Europe. The former appears to be a possibility, although pressure on cost may shift production further afield. The latter carries with it a quality risk, despite manufacturers' willingness to guarantee quality. Overall, it appears that the long-term implications of lower prices achieved through tendering practices will be a (further) contraction of generic manufacturing and marketing activities in Europe and a shift of manufacturing outside Europe.
As far as other stakeholders are concerned, it appears that patients are willing to accept the outcome of tenders and their potential "side effects", which can create
confusion, but this would require continued information from the side of health insurers. Prescribers also need to be better informed about likely changes to products available. The low prices achieved through tender practices do mean that health insurers appropriates the vast majority of producer surplus, which, in turn, implies that resources are not available to pay for distribution or to discount prices at retail level. Pharmacies can lose part of their income in this case, whilst at the same time logistical expenses and transaction costs rise and they need to have closer interaction with patients to explain likely differences between products, when patients fill a new prescription. Ensuring that pharmacy income levels are not adversely affected is one of the necessary conditions to guarantee that the system retains an element of stability.
An additional dimension in this context – the impact of which is unknown and unpredictable at the moment – is the potential desire by other EU Member States to implement comparable practices for procuring (off-patent) medicines in their outpatient markets. The more countries decide to do so, the faster the precipitation to an environment whereby a European generics industry will cease to exist in its current form.
8. Concluding remarks
The introduction of tenders/rebate contracts in the Netherlands and Germany over the past 4-7 years has been characterised by fast uptake, increased implementation, and relative diversification in terms of the types of tendering models used. In the majority of cases, the one-company-wins-all applies in both the Netherlands and Germany, whereas in Germany, there may be up to three companies that are allowed to supply the market.
In the Netherlands, the preference policy applies in parallel with other measures implemented by health insurers, whereas in Germany, the rebate contracts apply to over 55% of the off-patent market and the rate is increasing.
Tenders for out-patient pharmaceuticals present an attractive option for payers/health insurers because they are shown to drive prices close to marginal cost and, if sustained over successive tender cycles, savings are achieved and retained as a result. The submission of the tender implies that discounting practices at retail level are eliminated and that on aggregate, health insurers are in complete control of the procurement process. Some of the stakeholders may be affected either because of greater logistics spending and discount elimination (pharmacy), or because of the requirement to explain to patients any change in the drug prescribed (both pharmacy and physician). There is some confusion among patients if the drug changes at different intervals, but there is no evidence that this is creating problems with adherence or that it leads to underuse or even, misuse of medicines.
As a result of the increased implementation and fast uptake of tenders, the environment for manufacturers has become very challenging in a number of ways: first, prices have been compressed down to marginal cost; second, due to price reductions and the increased implementation of the rebate contracts, changes in the business models and the overall market structure have appeared. Third, several manufacturers have diversified their portfolio in order to stay on the market. Finally, regulatory, legal and quality challenges remain.
References
Blackmore, T. (2005). Sole supply of influenza vaccine: economic common sense
or a disaster waiting to happen? New Zealand Medical Journal, 118(1219),
Carradinha, H. (2009). Tendering short-term pricing policies and the impact on
patients, governments and the sustainability of the generic medicines
industry. Journal of Generic Medicines, 6(4), 351–361.
Cheraghi, T. (2009). Umsetzung von Arzneimittel-Rabattverträgen in der Apotheke
– Eine Untersuchung zur Compliance und Versorgungsqualität auf Basis einer
Patientenbefragung. University of Applied Sciences, Cologne.
Cherici, C., Frazier, J., & Feldman, M. (2011). Navigating Drug Shortages in
American Healthcare: A Premier Healthcare Alliance Analysis. Premier Inc.,
March. Accessed 22nd Feb. 2012:
Dylst, P., Vulto, A., & Simoens, S. (2011). Tendering for outpatient prescription
pharmaceuticals: What can be learned from current practices in Europe?
Health policy, 101(2), 146–52.
EGA. (2011). Biosimilars Handbook, (2nd Edition).
Foundation for Pharmaceutical Statistics. (2010). SFK Facts and Figures 2010-
2009 in numbers. The Hague. 2012 English version available at:
Gatesman, M. L., & Smith, T. (2011). The shortage of essential chemotherapy
drugs in the United States. New England Journal of Medicine, 365, 1653–
Gröber-Grätz, D., & Gulich, M. (2010). Auswirkungen der Medikamenten-
Rabattverträge auf die Arzneitherapie im hausärztlichen Setting. Z f Allg
Med, 86, 7–8.
Hoffmann, F., Glaeske, G., & Pfannkuche, M. (2009). The Effect of Introducing
Rebate Contracts to Promote Generic Drug Substitution, on Doctors'
Prescribing Practices. Deutsches Arzteblatt Int, 106(48), 783–8.
IGES. (2011). Generika in Deutschland: Wettbewerb foerdern - Wirtschaftlichkeit
staerken, Berlin, 18th October.
IMS. (2010). Generic medicines: Essential contributors to the long-term health of
Institut für Demoskopie Allensbach. (2009). Gesundheits- und
Arzneimittelversorgung in der deutschen Bevölkerung. Eine
Repräsentativbefragung der Bevölkerung ab 16 Jahre. Umfrage 10042.
Kanavos, P., Seeley, E., & Vandoros, S. (2009). Tender systems for outpatient
pharmaceuticals in the European Union: Evidence from the Netherlands,
Germany and Belgium. EMINet Report. Accessed Feb. 2012:
Kostev, K., Fuchs, S., Bauer, C., & May, U. (2011). The negative impact of rebate
contracts on the health care of patients with depression in Germany. Int J
Clin Pharmacol Ther, 49(6), 397–402.
MEDIQ. (2012). Mediq announces restructuring Pharmacies in the Netherlands.
Press Release. Retrieved October 5, 2012:
MacKay, P. (2005). Is PHARMAC's sole-supply tendering policy harming the
health of New Zealanders. NZ Med J, 118, 1214.
May, U., Kötting, C., & Cheraghi, T. (2010). Non-Compliance als
gesundheitspolitische Nebenwirkung-Demoskopie und Problemanalyse am
Beispiel der Rabattverträge. Pharmazeutische Zeitung online. Retrieved from
Natz, A. (2008). Rebate contracts: risks and chances in the German generic
market. Journal of Generic Medicines, 5(2), 99–103.
Pharmaceutical Management Agency (2011). PHARMAC and medicines
purchasing 2011. Retrieved from:
Pharmaceutische Weekblad. (2012). 149(37), 9. Based on data from Foundation
for Pharmaceutical Statistics.
Quinzler, R., Bertsche, T., Szecsenyi, J., & Haefeli, W. (2008). Teilung von
Tabletten: Welchen Einfluss haben die Rabattverträge auf die
Verordnungsqualität? Medizinische Klinik, 103(8), 569–574.
Rücker, D. (2008). Rabattverträge: Einsparungen unter Zielpreisniveau.
Pharmazeutische Zeitung, 12. Retrieved from http://www.pharmazeutische-
US Department of Health and Human Services. (2011). A review of FDA's
approach to medical product shortages. Retrieved from
US Food and Drug Administration. Current drug shortages. Retrieved from
Van Haeren, E., Arickx, F., Soete, E., & Bormans, V. (2009). Public tendering fo off
patent pharmceuticals in Belgium - the Simvastatin case. Value in Health,
12(7), A343.
Wilson, D. (2012). Deepening Drug Shortages. Health Affairs, 31(2), 263–266.
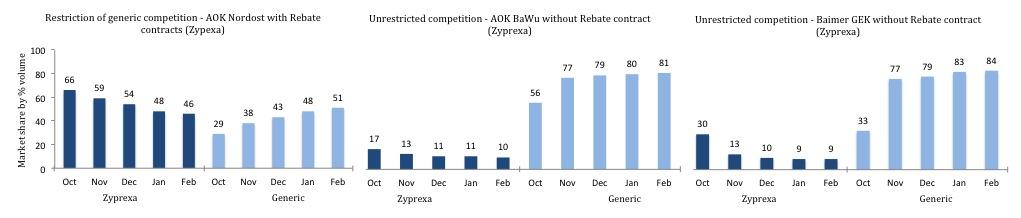
Appendix 1: Germany: Market share by % volume for Zyprexa 5 months post patent expiry (September 2011) across three regions,
illustrating effect of presence or absence of rebate contract on generic entry during October 2011-Feb. 2012.
Source: Pro Generika communication, 2012.
Source: http://www.zdravotnipojistenci.cz/static/soubory/clanek-26/ecdgenterprisednlpharma-14.pdf
Logo 28_issue 4_en.pdf
ISSUE 4 2012 CAMLOG Partner Magazine THE NEW CAMLOG APP FOR THE TITLE STORY Mobile end devices have long since found their way into the dental practice and for good reason. They inspire through technology and design and provide effective support in many work situations on demand. But there is more to it than just the end device: The right app
Harvard graphics - cora.prs
LE COMMERCE EN FRANCE GROUPE LOUIS DELHAIZE- PROVERA FRANCE LES AUTRES ENSEIGNES DU GROUPE LE COMMERCE EN FRANCE Cible des " gros", Cora s'en est toujours sortie : " attaquée" successivement par Carrefour puis par Casino, tous deux entrés au capital à hauteur de 42%, respectivement entre 1996 et 2001 et entre 2001 et 2006, Cora les a fait plier, pour conserver, aujourd'hui, 100% de son indépendance