Ui-tei.rnai.jp

Supplemental Material can be found at:http://www.jbc.org/content/suppl/2007/07/24/M703810200.DC1.html
THE JOURNAL OF BIOLOGICAL CHEMISTRY VOL. 282, NO. 37, pp. 27503–27517, September 14, 2007
2007 by The American Society for Biochemistry and Molecular Biology, Inc.
Printed in the U.S.A.
Cellular Internalization of Green Fluorescent Protein Fused
with Herpes Simplex Virus Protein VP22 via a Lipid
Raft-mediated Endocytic Pathway Independent
of Caveolae and Rho Family GTPases but
Dependent on Dynamin and Arf6*□S
Received for publication, May 9, 2007, and in revised form, July 2, 2007 Published, JBC Papers in Press, July 20, 2007, DOI 10.1074/jbc.M703810200
Kenji Nishi1 and Kaoru Saigo2
From the Department of Biophysics and Biochemistry, Graduate School of Science, University of Tokyo, 7-3-1 Hongo, Bunkyo-ku,
Tokyo 113-0033, Japan
VP22 is a structural protein of the herpes simplex virus and
showed that these short peptide-type PTDs and the proteins
has been reported to possess unusual trafficking properties.
associated with them are most likely to be incorporated into
Here we examined the mechanism of cellular uptake of VP22
cells via some endocytic mechanism (7, 8).
using a fusion protein between the C-terminal half of VP22 and
Endocytosis is a complex mechanism involving numerous
green fluorescent protein (GFP). Adsorption of VP22-GFP onto
protein-protein and protein-lipid interactions (9) and may
a cell surface required heparan sulfate proteoglycans and basic
occur as clathrin-dependent endocytosis, macropinocytosis,
amino acids, in particular, Arg-164 of VP22. Inhibitor treat-
caveola-mediated endocytosis, and lipid raft-dependent/caveola-
ment, RNA interference, expression of dominant-negative
independent endocytosis (9, 10). TAT and TAT-PTD fusion pro-
mutant genes, and confocal microscopy all indicated that VP22-
teins are capable of interacting with negatively charged plasma
GFP enters cells through an endocytic pathway independent of
membrane (11, 12), but their endocytic mechanism differs consid-
clathrin and caveolae but dependent on dynamin and Arf6 activ-
erably depending on cell type or protein moieties associated with
ity. As with CD59 (a lipid raft marker), cell-surface VP22-GFP
TAT-PTD (12–18). In some cases, they are internalized via mac-
signals were resistant to Triton X-100 treatment but only par-
ropinocytosis (13, 14), whereas in other cases, they appear to be
tially overlapped cell-surface CD59 signals. Furthermore, unlike
incorporated into cells via caveola-dependent endocytosis (15, 16)
other lipid raft-mediated endocytic pathways, no Rho family
or clathrin-dependent endocytosis (12, 18). Endosomal escape of
GTPase was required for VP22-GFP internalization. Internal-
TAT-PTD fusion proteins may require endosome acidification
ized VP22 initially entered early endosomes and then moved to
and cytosolic Hsp90 activity (18 –20).
lysosomes and possibly recycling endosomes.
VP22 is a major component of the herpes simplex virus type
1 tegment and is situated between the capsid and envelope (21).
This component has been shown to be secreted from cells
Short peptides and protein domains capable of transducing
expressing it through a Golgi-independent mechanism (22).
cargo across the plasma membrane are called protein transduc-
The C-terminal half of VP22, residues 159 –301 (termed
tion domains (PTDs)3 (reviewed in Refs. 1 and 2). A short basic
VP22.C1), is highly homologous in sequence to counterparts of
peptide from the TAT protein, a transcription activator of
other ␣-herpes virus VP22 proteins and is considered to be
human immunodeficiency virus type 1 (3–5), and the third
associated with intercellular trafficking ability (23, 24).
helix of the Drosophila Antennapedia homeodomain (6) are
Although others have reported that a 34-amino acid C-terminal
widely used as short peptide-type PTDs. Recent experiments
region of VP22.C1 is necessary and sufficient for cellular inter-nalization (25), our results indicate that the entire 142-aminoacid region is involved in cellular uptake.4
* This work was supported in part by a special coordination fund for promot-
VP22.C1 has been noted to form complexes with fluorescein-
ing science and technology and by grants from the Ministry of Education,
labeled oligonucleotides and generate particles of 0.3–1 m in
Culture, Sports, Science and Technology of Japan (to K. S.). The costs of
diameter (24). These particles are efficiently taken up into cul-
publication of this article were defrayed in part by the payment of pagecharges. This article must therefore be hereby marked "advertisement" in
tured cells or mice and release active antisense oligonucleotide
accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
reagents into the cytoplasm in response to light activation (24,
S The on-line version of this article (available at http://www.jbc.org) contains
26, 27). The uptake of VP22.C1-fused green fluorescent protein
supplemental Figs. 1–3.
1 To whom correspondence may be addressed. Tel.: 81-3-5841-3044; Fax:
(GFP), hereafter referred to as VP22-GFP, into an entire tumor
has been observed following peritumoral injection into human
2 To whom correspondence may be addressed. Tel.: 81-3-5841-3044; Fax:
pancreatic tumors in SCID mice (28). VP22.C1-Rab9 has been
3 The abbreviations used are: PTDs, protein transduction domains; GFP, green
reported to be internalized into cells and to exhibit biological
fluorescent protein; MCD, methyl--cyclodextrin; HA, hemagglutinin;
activity (29). Thus, as with short peptide-type TAT-PTD, the
DMEM, Dulbecco's modified Eagle's medium; PBS, phosphate-bufferedsaline; RNAi, RNA interference; siRNAs, small interfering RNAs; GAG,glycosaminoglycan.
4 K. Nishi and K. Saigo, unpublished data.
SEPTEMBER 14, 2007 • VOLUME 282 • NUMBER 37
JOURNAL OF BIOLOGICAL CHEMISTRY 27503

Supplemental Material can be found at:http://www.jbc.org/content/suppl/2007/07/24/M703810200.DC1.html
VP22 Internalization via Lipid Raft-mediated Endocytosis
142-amino acid VP22.C1 protein may possibly serve as a vector
GCGCCAACCCGATCCAA-3⬘ and 5⬘-CGGGTTAGATCT-
for delivering cargo into cells. As in the case of TAT-PTD and
CAATGGTGATGGTGATGATGAC-3⬘. PCR-based system-
its fusion proteins, VP22.C1 fusion proteins may also enter a
atic mutagenesis was carried out, and a series of pVP22-GFP
cell via endocytosis because they exhibit cytoplasmic vesicular
derivatives encoding mutants of VP22-GFP were generated.5
distribution (8). To date, the cellular internalization mecha-
pGFP, a GFP expression plasmid, was constructed by inserting
nism of VP22 fusion proteins is but little understood.
the 0.6-kb EcoRI/NotI fragment of pEGFP-N2 (Clontech) into
In this work, we studied the mechanism of cellular internal-
the EcoRI/NotI site of pET28a.
ization of VP22 using VP22-GFP as a model system. VP22-GFP
The pcDNA3-HA-dynamin-2a-WT and pcDNA3-HA-
was internalized through interactions with cell-surface heparan
dynamin-2a-K44A constructs (30) were obtained from Kazuhisa
sulfate proteoglycans and subsequent lipid raft-mediated endo-
Nakayama (Kyoto University). The pcDNA3-myc-Rab5-WT and
cytosis independent of clathrin, caveolae, and Rho family
pcDNA3-myc-Rab5-Q79L constructs (31) were kindly provided
GTPases but dependent on dynamin and Arf6 (ADP-ribosyla-
by Harald Hirling (Faculte´ des Science de la Vie). pRK5-myc-Rac1-
tion factor 6). Also, as with transferrin internalized via clathrin-
T17N was obtained from Gary Bokoch (Scripps Research Insti-
dependent endocytosis, nearly all internalized VP22-GFP sig-
tute). The pXS-HA-Arf6-Q67L plasmid (32) was kindly provided
nals were incorporated into the early endosomes and
by Julie G. Donaldson (National Institutes of Health).
co-localized with transferrin.
Protein Purification—VP22-GFP and GFP were produced
using E. coli BL21 Star (DE3) cells (Invitrogen) as host. Cells
were first grown overnight in L-broth with kanamycin at 37 °C.
Drugs and Antibodies—Alexa Fluor 647-labeled transferrin,
The overnight culture was diluted 10 times with the same
phalloidin-labeled rhodamine, and Hoechst 33342 were pur-
medium, and shaking was continued at room temperature. Iso-
chased from Molecular Probes. Heparin (porcine intestinal
propyl -D-thiogalactopyranoside was added to a final concen-
mucosa), chlorpromazine hydrochloride, methyl--cyclodex-
tration of 1 mM at A
⫽ 0.8–1.0. After an additional 12 h of
trin (MCD), genistein, and Clostridium difficile toxin B were
shaking at room temperature, the cell cultures were cooled on
obtained from Sigma. Cytochalasin D, okadaic acid, PP2, and
ice, and the cells were collected by centrifugation at 4 °C. The
sodium orthovanadate were from Calbiochem, and chloro-
pellets were frozen and stored at ⫺20 °C until used. Cells were
quine diphosphate salt was from Wako. Chondroitin sulfate A
thawed on ice, resuspended in lysis buffer (50 mM sodium phos-
(whale cartilage), chondroitin sulfate B (pig skin), chondroitin
phate (pH 8.0) containing 10 mM imidazole and 300 mM NaCl),
sulfate C (shark cartilage), keratan sulfate (bovine cornea), hep-
and disrupted by sonication. The lysate was centrifuged at
aritinase, and chondroitinase ABC were from Seikagaku Corp.
12,000 ⫻ g for 1 h at 4 °C. The supernatant was loaded onto a
The antibodies used were as follows: anti-heparan sulfate
nickel-nitrilotriacetic acid column (Qiagen Inc.) equilibrated
monoclonal antibody (clone NAH46; Seikagaku Corp.); rabbit
with lysis buffer. The column was washed with lysis buffer and
anti-hemagglutinin (HA) tag polyclonal IgG and goat anti-
then wash buffer (50 mM sodium phosphate (pH 8.0) containing
mouse IgM rhodamine (Upstate); mouse anti-c-Myc mono-
20 mM imidazole and 300 mM NaCl) and finally eluted with
clonal IgG (Oncogene); anti-Myc tag polyclonal antibody (Cell
elution buffer (50 mM sodium phosphate (pH 8.0) containing
Signaling Technology); mouse anti-human clathrin heavy
250 mM imidazole and 300 mM NaCl). Purified protein was
chain monoclonal antibody (Affinity BioReagents); anti-CD71
immediately used or frozen in 10% glycerol and stored at
antibody (transferrin receptor; Santa Cruz Biotechnology, Inc.);
⫺80 °C until used.
rabbit anti-EEA1 polyclonal IgG (Upstate); mouse anti-EEA1
Cell Culture and Transfection—Wild-type CHO-K1 and
monoclonal antibody and anti-human CD107a (LAMP1) anti-
HeLa cells were cultured in Dulbecco's modified Eagle's
body (Pharmingen); anti-human CD59 monoclonal antibody
medium (DMEM) supplemented with 10% fetal calf serum at
(Cedarlane Laboratories); rabbit anti-caveolin-1 polyclonal
37 °C in 5% CO . Glycosaminoglycan-deficient (pgsA-745) and
antibody and mouse anti-GM130 monoclonal antibody (BD
heparan sulfate-deficient (pgsD-677) mutant CHO-K1 cells
Transduction Laboratories); Cy3- or Cy5-conjugated goat anti-
(American Type Culture Collection) were cultured in Ham's
mouse IgG (Amersham Biosciences); and fluorescein isothio-
F12K medium supplemented with 10% fetal calf serum at 37 °C
cyanate-conjugated goat anti-mouse, Cy5-conjugated goat
in 5% CO . Transfection was carried out using Lipofectamine
anti-rabbit, and Cy3-conjugated goat anti-rabbit IgG (Jackson
2000 (Invitrogen) according to the manufacturer's instructions.
Usually, cells were analyzed within the period of 16 – 48 h fol-
Plasmids and VP22 Mutagenesis—pVP22-GFP, an expres-
sion plasmid for VP22-GFP production in Escherichia coli cells,
Visualization of Internalized Proteins by Confocal Micro-
was constructed as follows. A 0.6-kb EcoRI/NotI fragment of
scopy—Cells were cultured in 12-well culture plates (Sumitomo
pEGFP-N3 (Clontech) was inserted into the EcoRI/NotI site of
Bakelite Co., Ltd.) on a glass coverslip. When cells were treated
the pVP22/myc-His plasmid (Invitrogen). A fragment encoding
with drug or protein (VP22-GFP, GFP, or transferrin), the cul-
the C-terminal half of VP22 (amino acids 159 –301) and full-
ture medium was replaced with fresh serum-free medium at the
length enhanced GFP was amplified from the plasmid by PCR;
protein and/or drug concentrations indicated. The cells were
digested with NheI and NotI; and inserted into the NheI/NotI
incubated for 1 h and then washed twice with ice-cold phos-
site of pET28a (Novagen), which is capable of producingrecombinant protein with an N-terminal polyhistidine tag. The
5 The sequences of the primers used for mutagenesis are available upon
following primers were used: 5⬘-AAATTTGCTAGCACG-
27504 JOURNAL OF BIOLOGICAL CHEMISTRY
VOLUME 282 • NUMBER 37 • SEPTEMBER 14, 2007

Supplemental Material can be found at:http://www.jbc.org/content/suppl/2007/07/24/M703810200.DC1.html
VP22 Internalization via Lipid Raft-mediated Endocytosis
phate-buffered saline (PBS). After being fixed with 2– 4%
tion. A projection image was constructed using successive opti-
paraformaldehyde in PBS for 15 min at room temperature
cal sections of 0.25 m each. A region of interest was drawn
unless indicated otherwise, the cells were washed twice with
around each cell, and the average intensity was calculated using
ice-cold PBS and mounted in VECTASHIELD mounting
the software that came with the confocal microscope.
medium (Vector Laboratories). This was followed by cell visu-
Immunofluorescence—Cells were cultured in 12-well culture
alization with an Olympus Fluoview FV1000 confocal micros-
plates on a glass coverslip, incubated with protein at the speci-
copy system. Successive 0.25-m optical sections were taken
fied concentrations in serum-free DMEM for the indicated
using a ⫻60 objective lens. In some cases, after cells had been
times, washed twice with ice-cold PBS, and fixed with 4%
incubated on ice, the medium was replaced with fresh medium
paraformaldehyde at room temperature for 15 min. After being
containing protein(s) at the indicated concentrations. The cells
washed twice with ice-cold PBS, the cells were permeabilized
were then placed on ice for 1 h, washed twice with serum-free
with PBS containing 0.2% Triton X-100 and washed twice with
medium, placed in prewarmed serum-free medium, incubated
ice-cold PBS. Nonspecific binding sites were blocked with PBS
for a specified period of time, and then visualized as described
containing 5–10% goat whole serum (IBL Medical Products
above. Transferrin internalization was examined by exposing
Co.) at room temperature for 30 min. Following incubation
cells to culture medium containing 0.25 M Alexa Fluor 647-
with the primary antibody (1:100 – 400 dilution) in PBS con-
labeled transferrin and observing stained cells under a confocal
taining 1–5% goat whole serum for 1 h at room temperature or
overnight at 4 °C, the cells were washed twice with PBS and
Quantification of Internalized VP22-GFP—Cells were seeded
treated with the secondary antibody (1:100 – 400 dilution) in
on each well of 12-well culture plates in culture medium con-
PBS containing 1–5% goat whole serum for 1 h at room tem-
taining 10% fetal calf serum. Culturing was continued until pre-
perature or overnight at 4 °C. This was followed by two washes
confluency at 37 °C. The medium was replaced with fresh
with PBS and visualization by confocal microscopy as described
serum-free medium containing 1 M VP22-GFP. Cells were
incubated for the indicated times or for 1 h, washed once with
RNA Interference (RNAi)—Two sets of human clathrin heavy
ice-cold PBS, and trypsinized for 15 min at 37 °C to eliminate
chain small interfering RNA (siRNAs) were selected using
non-internalized cell-surface protein. This was followed by
siDirect (33): CHC1 (clathrin heavy chain 1), GCUUCAGUAC-
centrifugation in 1.5-ml tubes, and the cells were washed three
CCUGACUAUGG (passenger strand) and AUAGUCAGGGU-
times with ice-cold PBS, lysed with PBS containing 0.5% Triton
ACUGAAGCCA (guide strand); and CHC2, CCUGGUACGU-
X-100, and disrupted by sonication. The cell lysate was centri-
CGAAAGGAUCC (passenger strand) and AUCCUUUCGAC-
fuged. The protein content of the supernatant was measured
GUACCAGGUA (guide strand). Firefly luciferase siRNA was
using a Bio-Rad protein assay kit with bovine serum albumin as
used as a control: CGUACGCGGAAUACUUCGAAA (passen-
the standard. Fluorescence intensity was measured with a Hita-
ger strand) and UCGAAGUAUUCCGCGUACGUG (guide
chi FL-2500 spectrofluorometer at a excitation wavelength of
strand). RNA oligonucleotides were synthesized by Proligo, and
488 nm and a emission wavelength of 511 nm. Replacement of
double-stranded siRNA was prepared as described previously
0.5% Triton X-100 with 0.1% SDS gave no appreciable differ-
(34). HeLa cells in 12-well plates were transfected three times at
ence in measurement, indicating that almost all internalized
24 –36-h intervals with 50 nM siRNA. Firefly luciferase siRNA-
VP22-GFP is solubilized by 0.5% Triton X-100 treatment.
treated and CHC siRNA-treated cells were trypsinized, mixed
Low Temperature Treatment and ATP Depletion—For low
at a ratio of 1:1, and seeded in 12-well plates 20 h prior to
temperature treatment, after being incubated at 4 °C for 1 h, the
observation of the effects of clathrin heavy chain depletion in
cells were further incubated in the presence of VP22-GFP for
the same field. DNA transfection was conducted with a third
1 h at 4 °C and subjected to trypsin digestion or fixation. For
siRNA transfection.
removal of cellular ATP, the cells were incubated with ATP
DNA-mediated RNAi was also carried out to knock down the
depletion medium (glucose-free DMEM with 10 mM sodium
genes encoding RhoA, Cdc42, and Arf6. A firefly luciferase
azide and 6 mM 2-deoxy-D-glucose) containing 10% fetal calf
short hairpin RNA expression construct was used as a control.
serum for 1.5 h, followed by incubation in the presence of the
Details of vector construction will be described elsewhere.6
indicated concentrations of VP22-GFP for 1 h in serum-free
HeLa cells in 12-well plates were transfected with a suitable
ATP depletion medium. Cellular protein uptake was visualized
construct, and transfectants were selected with puromycin (2
or measured as described above.
g/ml) 24 h after transfection. Cells were analyzed 96 h after
Quantification of Cell-surface VP22-GFP and Anti-heparan
Sulfate Antibody Binding Signals—Cells were cultured in
Drug Treatment—Cells were washed twice with serum-free
12-well culture plates on a glass coverslip. Cells were washed
DMEM and incubated in serum-free DMEM containing a given
twice with serum-free DMEM and incubated on ice for 1 h.
drug at the indicated concentrations for 30 min except for
After addition of the indicated concentrations of VP22-GFP or
C. difficile toxin B. Following protein addition to the medium,
2 g/ml anti-heparan sulfate antibody in serum-free DMEM,
cells were incubated for 1 or 4 h. Protein uptake and cell-surface
cells were further incubated on ice for 1 h, washed twice with
binding were visualized and measured as described above.
ice-cold PBS, fixed with 4% paraformaldehyde at room temper-
Triton X-100 Treatment—After being put on ice with 0.25
ature for 15 min, and visualized by confocal microscopy. For
M VP22-GFP, 2 g/ml anti-CD59 antibody, or 1 g/ml anti-
anti-heparan sulfate antibody staining, the secondary antibodytreatment was also carried out before paraformaldehyde fixa-
6 K. Ui-Tei, K. Nishi, and K. Saigo, unpublished data.
SEPTEMBER 14, 2007 • VOLUME 282 • NUMBER 37
JOURNAL OF BIOLOGICAL CHEMISTRY 27505

Supplemental Material can be found at:http://www.jbc.org/content/suppl/2007/07/24/M703810200.DC1.html
VP22 Internalization via Lipid Raft-mediated Endocytosis
transferrin receptor antibody on ice for 1 h, cells were washedtwice with serum-free DMEM, stained with the secondary anti-body for 1 h in serum-free DMEM, washed twice with ice-coldPBS, treated with 1% Triton X-100 in PBS for 7 min, and washedtwice again with ice-cold PBS on ice. They were then fixed with4% paraformaldehyde and visualized.
Cell Treatment with Glycosaminoglycan (GAG) Lyases—En-
zymatic treatment with GAG lyases was carried out as reportedpreviously (35). Briefly, preconfluent cells were washed oncewith PBS, followed by incubation with GAG lyases in PBS con-taining 0.1% bovine serum albumin, 0.2% gelatin, and 0.1% glu-cose for 2 h at 37 °C. Four washings with PBS or serum-freeDMEM were carried out. Following addition of the indicatedconcentrations of VP22-GFP to the medium, cells were incu-bated for 1 h. VP22-GFP uptake and binding were visualizedand measured as described above.
Real-time PCR—The total RNA from transfected cells was
isolated using an RNeasy mini kit (Qiagen Inc.). The RNA wastreated with RQ1 DNase (Promega Corp.), purified using theRNeasy mini kit, and reverse-transcribed using SuperScript III(Invitrogen). Before the PCR, a mixture of the synthesizedcDNA and SYBR Green PCR Master Mix (Applied Biosystems)
was incubated at 95 °C for 10 min. PCR was then carried out at95 °C for 15 s and at 60 °C for 1 min for 40 cycles. All reactionswere carried out in triplicate. The levels of PCR products were
monitored with an ABI PRISM 7000 sequence detection systemand analyzed with ABI PRISM 7000 SDS software (Applied Bio-systems). For each sample, the amount of target mRNA wasnormalized using endogenous -actin mRNA. The followingprimers were used: Arf6, 5⬘-ATCAATGACCGGGAGAT-GAG-3⬘ (forward) and 5⬘-AGGGCTGCACATACCAGTTC-3⬘(reverse); Cdc42, 5⬘-CGATGGTGCTGTTGGTAAAA-3⬘ (for-ward) and 5⬘-TCCTCTTGCCCTGCAGTATC-3⬘ (reverse);RhoA, 5⬘-CGGTCTGGTCTTCAGCTACC-3⬘ (forward) and5⬘-CCATCACCAACAATCACCAG-3⬘ reverse; and -actin,5⬘-CACACTGTGCCCATCTACGA-3⬘ (forward) and 5⬘-GCC-ATCTCTTGCTCGAAGTC-3⬘ (reverse).
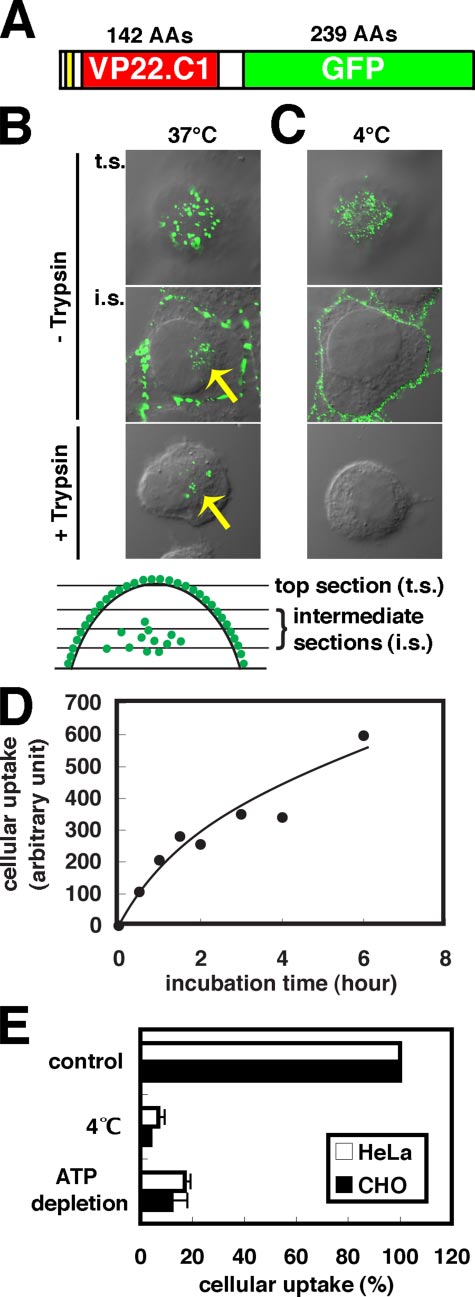
Cellular Uptake of VP22-GFP Occurs through Endocytosis—
To clarify the mechanism for the cellular uptake of VP22,VP22-GFP, a fusion protein between GFP and VP22.C1 (Fig.
1A), was prepared from E. coli cells and added to the culturemedium with HeLa cells. GFP signal distribution was examinedat 1 h of incubation at 37 °C using a confocal microscope (Fig.
1B). Significant VP22-GFP signals were observed not only on
before paraformaldehyde fixation. VP22-GFP signals (green) in the top andintermediate optical sections (see the lower margin) and a differential inter-ference contrast image were merged. Arrows indicate internalized VP22-GFPsignals. C, HeLa cells exposed to VP22-GFP at 4 °C. Note the absence of inter-nalized VP22-GFP signals. D, time course of intracellular accumulation ofVP22-GFP in HeLa cells at 37 °C. Cells were exposed to 1 M VP22-GFP. The
amount of intracellular VP22-GFP signals was measured spectrofluorometri-cally after trypsin treatment. Two measurements were averaged. E, effects of
FIGURE 1. Cellular VP22 uptake via endocytosis. A, structure of VP22-GFP.
low temperature and ATP depletion on cellular uptake of VP22-GFP in HeLa
The 6-amino acid histidine tag, VP22.C1, and GFP are shown in yellow, red, and
and CHO-K1 cells. HeLa and CHO-K1 cells were exposed to 1 M VP22-GFP at
green, respectively. AAs, amino acids. B, VP22-GFP signals in HeLa cells with
4 °C or under ATP depletion conditions. Three independent spectrofluoro-
(lower panel) or without (upper and middle panels) trypsin treatment. HeLa
metric measurements obtained after trypsin treatment were averaged. Error
cells were exposed to 0.5 M VP22-GFP at 37 °C for 1 h, fixed with paraform-
bars indicate S.D. The cellular uptake of VP22-GFP found in cells cultured
aldehyde, and observed under a confocal microscope. Cells were trypsinized
under normal conditions at 37 °C is also shown (control).
27506 JOURNAL OF BIOLOGICAL CHEMISTRY
VOLUME 282 • NUMBER 37 • SEPTEMBER 14, 2007

Supplemental Material can be found at:http://www.jbc.org/content/suppl/2007/07/24/M703810200.DC1.html
VP22 Internalization via Lipid Raft-mediated Endocytosis
the surface of the cells (Fig. 1B, top section (t.s.)) but also inside
pgsD-667 cells have been shown not to produce heparan sulfate
(intermediate sections (i.s.)). Fifteen minutes of trypsin treat-
but instead 3– 4 times as many chondroitin sulfate molecules as
ment at 37 °C eliminated almost completely the cell-surface
wild-type cells (38). Fig. 2C shows that cell-surface and inter-
signals without affecting intracellular signal intensity (Fig. 1B,
nalized VP22-GFP signals underwent significant reduction in
compare middle and lower panels). Cellular VP22-GFP uptake
pgsA-745 and pgsD-667 cells; thus, as with HeLa cells, heparan
appeared to depend on the VP22 moiety because no intracellu-
sulfate (but not chondroitin sulfates) is required for VP22-GFP
lar GFP signal was detected upon elimination of the entire
binding to the cell surface and internalization in CHO-K1 cells.
VP22.C1 moiety from the fusion protein (data not shown). Change
To further confirm the close relation between cell-surface
in intracellular GFP signal intensity was examined spectrofluoro-
VP22-GFP adsorption and heparan sulfate, we examined
metrically using trypsin-treated HeLa cells (Fig. 1D). The intracel-
whether the distribution of VP22-GFP signals on the cell sur-
lular VP22-GFP signal increased gradually for ⬎6 h.
face is in some way related to that of heparan sulfate signals (Fig.
Fig. 1B shows that the cytoplasmic distribution of VP22-GFP
2D). Anti-heparan sulfate antibody staining revealed that the
in HeLa cells is vesicular, supporting the notion that VP22-GFP
heparan sulfate on the surface of HeLa cells was punctate.
is internalized through endocytosis (8). Endocytosis-dependent
Nearly all punctate signals of heparan sulfate were found to
cellular internalization would not occur either at 4 °C or in the
co-localize with VP22-GFP signals that had distributed in a
absence of ATP (7). As shown in Fig. 1 (C and E), no or hardly
punctate fashion and vice versa (Fig. 2D). However, it should be
any intracellular accumulation of VP22-GFP could be detected
noted that heparan sulfate signal intensity was not always pro-
in HeLa cells exposed to VP22-GFP at 4 °C. In contrast to cel-
portional to VP22-GFP signal intensity, possibly suggesting
lular uptake, VP22-GFP adsorption onto the cell surface
involvement of other factors in cell-surface VP22-GFP
appeared to occur quite normally even at 4 °C. The cellular ATP
pool becomes empty upon preincubation of cells with sodium
Involvement of Basic Amino Acids in the VP22.C1 N-terminal
azide and deoxyglucose (36). Only slight cellular uptake of
Region in Cell-surface Adsorption and Intracellular Uptake of
VP22-GFP without significant reduction in surface signals was
VP22-GFP—As with TAT-PTD, VP22.C1 is rich in basic amino
apparent in both ATP-depleted HeLa and CHO-K1 cells (Fig.
acids (Fig. 2E) (23). Mutational analysis indicated that 8 basic
1E). VP22-GFP may thus be concluded to be internalized
amino acids in TAT-PTD are equally requisite for cellular
through endocytosis in HeLa and CHO-K1 cells.
uptake activity (39). To determine which basic amino acids of
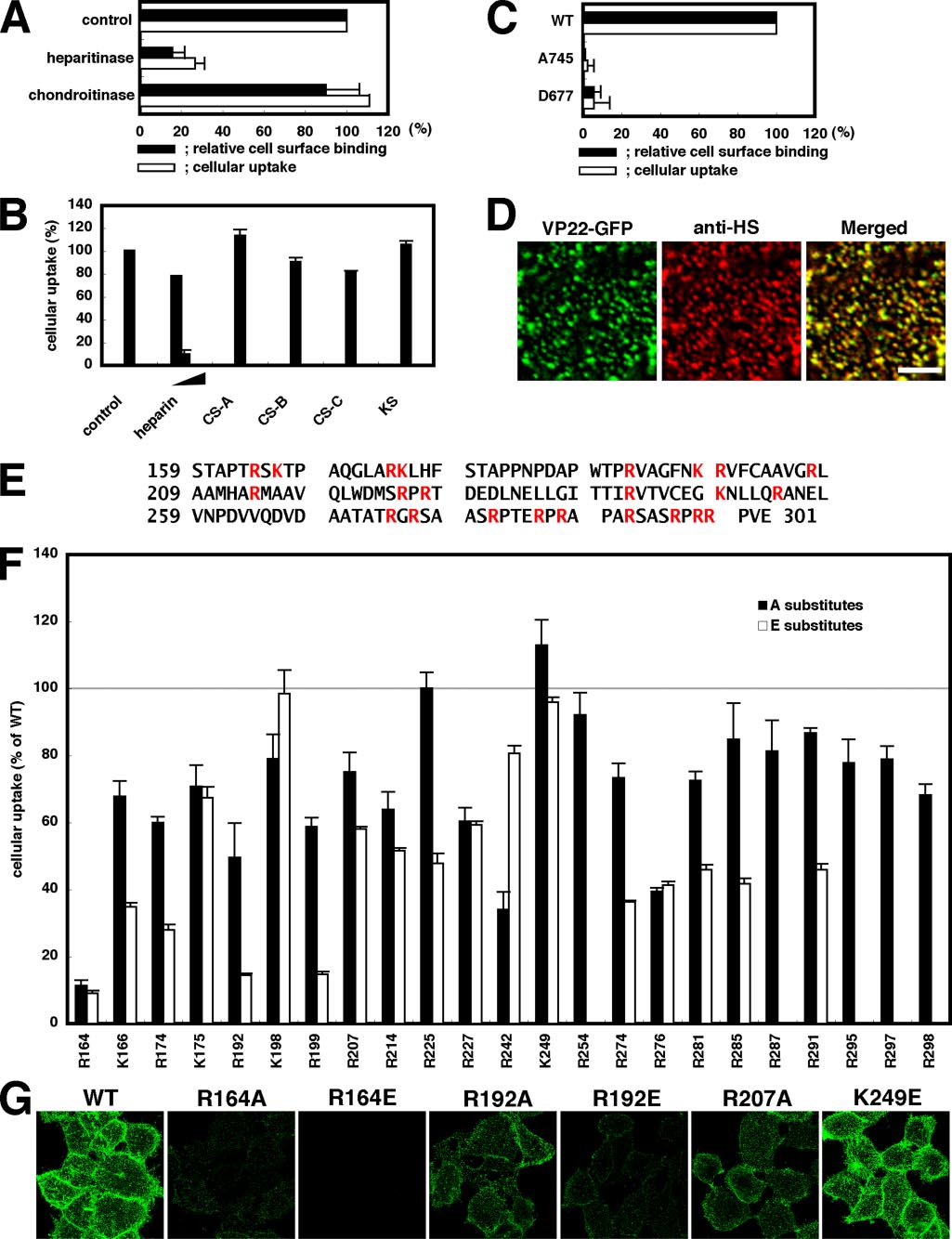
Requirement of Heparan Sulfate for Cell-surface Binding and
VP22.C1 are required for interactions between heparan sulfate
Cellular Uptake of VP22-GFP—Cellular internalization of
and VP22-GFP, the basic amino acids of VP22.C1 were system-
TAT-PTD requires proteoglycans (11, 12). In the case of mam-
atically replaced with alanine (neutral amino acid) or glutamic
malian cell-surface GAGs, heparan sulfate and chondroitin sul-
acid (acidic amino acid), and we examined spectrofluorometri-
fates A–C are the main components. We thus investigated
cally whether mutant VP22-GFP proteins were effectively
whether GAGs are required for cell-surface adsorption and cel-
incorporated into cells (Fig. 2F). The R164A and R164E mutant
lular uptake of VP22-GFP. After HeLa cells were treated with
proteins were found to lose 90% of the intracellular incorpora-
heparitinase or chondroitinase ABC, cell-surface adsorption
tion activity of the wild-type protein, indicating the importance
and cellular uptake of VP22-GFP signals were assessed. Hep-
of arginine at position 164 for VP22-GFP internalization. At
aritinase digests heparan sulfate, whereas chondroitinase ABC
positions 174, 192, and 199, only glutamic acid substitutes
digests chondroitin sulfates A–C but not heparan sulfate. Hep-
exhibited significant reduction in internalization activity, sug-
aritinase treatment eliminated not only cell-surface VP22-GFP
gesting that the absence of negative charges at these positions is
signals but cellularly internalized VP22-GFP signals as well (Fig.
required for effective intracellular uptake of VP22-GFP. Fig. 2F
2A). In contrast, virtually no reduction in VP22-GFP signals on
shows that most, if not all, other basic amino acids of VP22.C1,
either the surface of or inside cells could be detected by chon-
which are associated with moderate mutational effects, may
droitinase ABC treatment (Fig. 2A). GAGs were added to the
also be involved in VP22-GFP internalization to some extent.
culture medium, and only heparin (analog of heparan sulfate)
Reduction in VP22-GFP internalization activity appeared to
addition was found to induce a significant reduction in intra-
be virtually proportional to that in cell-surface binding activity.
cellularly accumulated VP22-GFP signals (Fig. 2B). Thus, in
Indeed, cell-surface VP22-GFP signals were almost completely
HeLa cells, heparan sulfate (but not chondroitin sulfates) is
abolished or significantly reduced in R164E, R164A, and R192E,
required for the surface adsorption and intracellular accumu-
three strong internalization-defective mutants, whereas cell-
lation of VP22-GFP.
surface VP22-GFP signals in R192A and R207A, associated
pgsA-745 and pgsD-677 cells are mutant GAG strains of
with moderate internalization defects, were moderately
CHO-K1 cells (37, 38). pgsA-745 is deficient in xylosyltrans-
reduced (Fig. 2, compare F and G).
ferase, the key enzyme required for GAG attachment to core
Requirement of Dynamin in Cellular Internalization of
protein. Only 1% of GAGs expressed in wild-type cells have
VP22-GFP—Dynamin is a GTPase that is essential for various
been reported to be expressed in pgsA-745 cells (37). In this
endocytic pathways (10). Cellular expression of the GTPase-
study, very few if any heparan sulfate signals could be detected
deficient, dominant-negative mutant of dynamin (dynamin-
by anti-heparan sulfate antibody staining in both pgsA-745 and
K44A) has no effect on macropinocytosis or certain lipid
pgsD-677 cells (supplemental Fig. 1). pgsD-667 is deficient in
raft-mediated endocytic pathways, including that induced
by Arf6-Q67L (32), but almost entirely blocks clathrin-medi-
both of which are essential for heparan sulfate polymerization.
ated endocytosis and other lipid raft-mediated endocytic path-
SEPTEMBER 14, 2007 • VOLUME 282 • NUMBER 37
JOURNAL OF BIOLOGICAL CHEMISTRY 27507

Supplemental Material can be found at:http://www.jbc.org/content/suppl/2007/07/24/M703810200.DC1.html
VP22 Internalization via Lipid Raft-mediated Endocytosis
27508 JOURNAL OF BIOLOGICAL CHEMISTRY
VOLUME 282 • NUMBER 37 • SEPTEMBER 14, 2007
Supplemental Material can be found at:http://www.jbc.org/content/suppl/2007/07/24/M703810200.DC1.html
VP22 Internalization via Lipid Raft-mediated Endocytosis
ways such as caveola-mediated endocytosis (10, 40 – 43). Usingtransferrin as a dynamin-dependent endocytosis marker, weexamined VP22-GFP internalization in HeLa cells transfectedwith wild-type dynamin-2 or dynamin-2-K44A. Dynamin-overexpressing cells were identified by anti-HA antibody.
Overexpression of wild-type dynamin-2 resulted in virtually nochange in VP22-GFP and transferrin internalization (data notshown). In contrast the internalization of VP22-GFP and trans-ferrin was noted to be significantly reduced in nearly 60 and80% of cells overexpressing dynamin-2-K44A, respectively (Fig.
3, A and C). No apparent dynamin-2-K44A-dependent changein cell-surface heparan sulfate signals was observed (Fig. 3B).
Dynamin-2 is thus essential for the normal cellular internaliza-tion of VP22-GFP, and also, VP22-GFP may not be internalizedthrough macropinocytosis and lipid raft-mediated endocytosisinduced by Arf6-Q67L but instead by clathrin-mediated endo-
cytosis or dynamin-dependent lipid raft-mediated endocytosis,such as that mediated by caveolae. Note that heparan sulfate(Fig. 3B) as well as cell-surface VP22-GFP (Fig. 3D) signals werenot affected by the absence of dynamin activity, indicating thatdynamin is involved in cellular uptake, but not in VP22-GFPadsorption.
VP22-GFP Endocytosis Is Independent of Clathrin—By far,
clathrin-mediated endocytosis has been best characterized andhas been shown to be inhibited specifically by chlorpromazine
FIGURE 3. Requirement of dynamin for VP22-GFP endocytosis. A, HeLa
(44). Cell treatment with chlorpromazine induces clathrin-
cells transfected with pcDNA3-HA-dynamin-2a-K44A, an expression plasmidencoding an HA-tagged dominant-negative form of dynamin (K44A). Cells
coated pit misassembly at the plasma membrane, so clathrin-
were exposed to VP22-GFP (0.5 M) and Alexa Fluor 647-labeled transferrin
mediated endocytosis such as transferrin uptake is prevented to
(0.25 M) 24 h after transfection. Thick arrows show anti-HA antibody-stained
a significant degree in chlorpromazine-treated cells. To con-
K44A-expressing cells, and thin arrows show normal cells exhibiting intracel-lular accumulation of VP22-GFP and transferrin signals but lacking dynamin
firm any possible involvement of clathrin-mediated endocyto-
activity. B, normal accumulation of heparan sulfate on the surface of cells
sis in the cellular internalization of VP22, we examined the
lacking dynamin activity. Cell-surface heparan sulfate was detected by anti-
effects of chlorpromazine treatment on VP22-GFP internaliza-
heparan sulfate (anti-HS) antibody. Note that heparan sulfate signals werenot reduced in cells expressing dynamin-K44A. C, proportion of cells associ-
tion in HeLa cells (Fig. 4, A and B).
ated with internalized signals of VP22-GFP or Alexa Fluor 647-labeled trans-
Chlorpromazine treatment (10 g/ml, 30 min) significantly
ferrin (Tf). Measurements from two independent experiments were averaged(each case, n ⱖ 30). Error bars indicate S.D. D, cell-surface VP22-GFP signals in
reduced transferrin internalization in 60% of the cells (Fig. 4A,
K44A-expressing cells. HeLa cells transfected with pcDNA3-HA-dynamin-
arrows), whereas VP22-GFP internalization was actually
2a-WT or pcDNA3-HA-dynamin-2a-K44A were incubated for 1 h on ice with fresh
enhanced in virtually all cells exhibiting a significant reduction
serum-free medium containing 0.25 M VP22-GFP. Surface signals were deter-
mined by confocal microscopy (n ⱖ 33). Error bars indicate S.D. WT, wild-type
in transferrin uptake (Fig. 4A). Clathrin-mediated endocytosis
thus apparently is not involved in VP22-GFP internalization inHeLa cells.
rin-depleted cells (45, 46). For observation of clathrin-depleted
For further clarification of the above, the CHC gene was
and non-depleted cells in the same field, a 1:1 mixture of CHC1
knocked down using RNAi because clathrin-mediated endocy-
siRNA-treated cells and firefly luciferase siRNA (control siRNA
tosis was reported previously to be severely inhibited in clath-
unrelated in sequence to the CHC gene)-treated cells was
FIGURE 2. Requirement of cell-surface heparan sulfate and VP22.C1 basic amino acids for cellular internalization of VP22-GFP. A, effects of heparitinase
or chondroitinase treatment on cell-surface binding and intracellular accumulation of VP22-GFP. HeLa cells were treated with 25 milliunits/ml heparitinase or
chondroitinase ABC and exposed to 0.25 M (cell-surface binding) or 1 M (intracellular accumulation) VP22-GFP for 1 h. Black bars, cell-surface VP22-GFP
signals measured using a confocal microscope (n ⱖ 23) (see "Experimental Procedures"); white bars, intracellular GFP signals measured spectrofluorometicallyafter trypsin treatment. Three independent measurements were averaged. Error bars indicate S.D. B, inhibition of intracellular VP22-GFP accumulation by GAGs.
Cells were exposed to 1 M VP22-GFP in medium containing 1–25 g/ml heparin (analog of heparan sulfate) and 25 g/ml chondroitin sulfate (CS) A, B, or C
or keratan sulfate (KS) for 1 h, and intracellular GFP signals were measured spectrofluorometically after trypsin treatment. Three independent measurementswere averaged. C, relative cell-surface binding (black bars) and intracellular accumulation (white bars) of VP22-GFP in wild-type (WT) and mutant (pgsA-745 andpgsD-677) CHO-K1 cells. Cell-surface binding (0.5 M VP22-GFP; n ⱖ 34) and intracellular accumulation (1 M VP22-GFP; six measurements) of VP22-GFP signals
were determined as described for A. D, co-localization of VP22-GFP and heparan sulfate signals on the HeLa cell surface. HeLa cells were incubated for 1 h onice with fresh serum-free medium containing 0.25 M VP22-GFP and 2 g/ml anti-heparan sulfate (anti-HS) antibody. The secondary antibody treatment was
carried out before paraformaldehyde fixation. A cell-surface section is shown. Scale bar ⫽ 5 m. E, amino acid sequence of VP22.C1. Ser159 of VP22 corresponds
to the N terminus of VP22.C1. Basic amino acids are shown in red. F and G, effects of the substitution of basic amino acids of VP22.C1 on intracellularaccumulation (1 M VP22-GFP; F) and cell-surface binding (0.25 M VP22-GFP; G) of VP22-GFP. Black bars, intracellular accumulation in alanine-substituted
mutants; white bars, intracellular accumulation in glutamic acid-substituted mutants. Intracellular accumulation of VP22-GFP signals was determined spec-trofluorometrically after trypsinization. Three measurements were averaged. R254E, R287E, R295E, R297E, and R298E are not tested. Cell-surface signals wereobserved under a confocal microscope (projection figures; G). Note that cell-surface VP22-GFP signals were significantly reduced in R164A-, R164E-, andR192E-exposed cells. R192A and R207A gave moderately reduced signals, whereas K249E gave virtually no reduction in signal intensity.
SEPTEMBER 14, 2007 • VOLUME 282 • NUMBER 37
JOURNAL OF BIOLOGICAL CHEMISTRY 27509
Supplemental Material can be found at:http://www.jbc.org/content/suppl/2007/07/24/M703810200.DC1.html
VP22 Internalization via Lipid Raft-mediated Endocytosis
Lipid Raft Dependence of Cellular Uptake of VP22-GFP—Dy-
namin-2 is required for certain lipid raft-mediated endocyticpathways such as caveola-mediated endocytosis. We thusexamined whether VP22-GFP uptake is dependent on lipidraft-mediated endocytosis. Lipid rafts are detergent (TritonX-100)-resistant domains rich in cholesterol and sphingolipids(48), and CD59 serves as a marker for a subgroup of lipid rafts(32). Cell-surface signals of not only CD59 but also VP22-GFPwere found to be resistant to Triton X-100 treatment (Fig. 5A),but virtually all transferrin receptor signals, internalized in alipid raft-independent manner, were completely eliminatedsubsequent to Triton X-100 treatment (Fig. 5A). Double stain-ing showed that cell-surface VP22-GFP signals partially over-lapped (if at all) CD59 signals in Triton X-100-treated HeLacells (Fig. 5B), suggesting that VP22 may be internalized byendocytosis in a different way compared with CD59. Consistent
with this, most intracellular VP22-GFP signals were found notto be co-localized with internalized CD59 signals at early stages(Fig. 5C and supplemental Fig. 2A), although at late stages inparticular, in most cells expressing Arf6-Q67L, VP22-GFP sig-nals were co-localized with large CD59-positive Arf6-Q67L-induced vesicles (Fig. 5D) (32). Cell-surface heparan sulfate-
binding proteins such as lipoprotein lipase were shownpreviously to be resistant to Triton X-100 treatment (49), indi-cating the presence of heparan sulfate on the surface of Triton
MCD disrupts lipid rafts (50). Genistein is a tyrosine kinase
inhibitor that prevents lipid raft-mediated endocytosis such ascaveola-dependent endocytosis (50 –52). Consistent with thenotion that VP22-GFP is internalized through lipid raft-medi-ated endocytosis, intracellular VP22-GFP signals in HeLa cellswere significantly reduced subsequent to MCD (Fig. 5E) orgenistein (Fig. 5F) treatment. MCD treatment did not change
FIGURE 4. Clathrin-independent cellular uptake of VP22-GFP. A, stimula-
the intensity of cell-surface heparan sulfate signals, whereas a
tion of intracellular VP22-GFP accumulation by chlorpromazine treatment.
considerable heparan sulfate signal was abolished by genistein
Lower panels, HeLa cells treated with 10 g/ml chlorpromazine for 30 min
were exposed to 0.5 M VP22-GFP and 0.25 M Alexa Fluor 647-labeled trans-
treatment (Fig. 5H), suggesting that genistein may prevent
ferrin at 37 °C for 1 h. Arrows show cells with reduced transferrin uptake. In
VP22-GFP internalization via reduction in cell-surface heparan
these cells, intracellular VP22-GFP signals significantly increased, indicatingthat clathrin is not involved in cellular uptake of VP22-GFP. B, quantitative
data for chlorpromazine-dependent intracellular VP22-GFP signal accumula-
The actin cytoskeleton is required for lipid raft-mediated
tion. After chlorpromazine treatment, cells were exposed to 1 M VP22-GFP
endocytosis such as that mediated by caveolae (52). Upon treat-
for 1 h. Measurements from three experiments were averaged. Error bars indi-cate S.D. C, effect of CHC1 RNAi. RNAi for the clathrin heavy chain was carried
ment of HeLa cells with cytochalasin D, an actin-depolymeriz-
out using CHC1 siRNA. Thick arrows show clathrin-negative cells in which
ing reagent, actin filament disruption occurred, with conse-
internalized transferrin (but not VP22-GFP) signals were abolished, and thin
quent large membrane bleb (Fig. 5G) or protrusion (53)
arrows show wild-type cells with clathrin production. D, proportion of cellsassociated with internalized signals of VP22-GFP or Alexa Fluor 647-labeled
formation on the cell surface. Virtually all cell-surface VP22-
transferrin (Tf) in anti-clathrin antibody-positive (Clathrin⫹) or -negative
GFP signals appeared to be co-localized with membrane blebs
(Clathrin⫺) cells. Measurements from two independent experiments wereaveraged (each case, n ⱖ 50). Errors bars indicate S.D.
induced by cytochalasin D (Fig. 5G). A similar phenomenon hasbeen reported to occur in the case of the Helicobacter pylori
plated. In clathrin-negative cells, transferrin uptake was almost
vaculating cytotoxin VacA (53). In cytochalasin D-treated cells
completely inhibited, whereas VP22-GFP uptake was essen-
manifesting these membrane blebs, the cellular uptake of
tially the same as that in clathrin-positive normal cells (Fig. 4, C
VP22-GFP was significantly reduced (Fig. 5, F and G) without
and D); but unlike the case of chlorpromazine treatment, no
reduction in cell-surface heparan sulfate signals (Fig. 5H). It
apparent enhancement of VP22-GFP uptake could be detected
may thus follow that VP22-GFP is internalized via lipid raft-
(Fig. 4, compare A and C). Essentially the same was noted with
RNAi using a different CHC siRNA (CHC2) (data not shown),
Caveolae Are Not Involved in VP22-GFP Internalization—To
indicating that the siRNA-induced change is not due to the
determine whether caveolae are involved in VP22-GFP inter-
off-target effects (47) but instead to the knockdown of clathrin
nalization, we examined the possible requirement of caveolae
gene activity. The cellular uptake of VP22-GFP may thus be
for the cellular internalization of VP22-GFP. In cells other than
concluded to be independent of clathrin-mediated endocytosis.
those derived from muscle, caveolin-1 is a major structural
27510 JOURNAL OF BIOLOGICAL CHEMISTRY
VOLUME 282 • NUMBER 37 • SEPTEMBER 14, 2007
Supplemental Material can be found at:http://www.jbc.org/content/suppl/2007/07/24/M703810200.DC1.html
VP22 Internalization via Lipid Raft-mediated Endocytosis
FIGURE 5. Requirement of lipid rafts for VP22 endocytosis. A, HeLa cells exposed to VP22-GFP, anti-CD59 antibody, or anti-transferrin receptor (Tf-R)
antibody were treated with Triton X-100. Projection images of successive 0.25-m optical sections are shown. The cell-surface signals of VP22-GFP were
resistant to Triton X-100 treatment, whereas those of the transferrin receptor were completely eliminated. PBS indicates the control. B–D, relationship betweencell-surface (B) and intracellular (C and D) signals of VP22-GFP and CD59. B, after HeLa cells exposed to 0.25 M VP22-GFP were treated with Triton X-100, cells
were stained with anti-CD59 antibody. A cell-surface section is shown. Scale bar ⫽ 5 m. C, cells were exposed to 0.5 M VP22-GFP and 2 g/ml anti-CD59
antibody on ice for 1 h, washed, incubated at 37 °C for 15 min, fixed with 4% paraformaldehyde, and stained with secondary antibody. In the Merged panels,VP22-GFP and CD59 signals are shown in green and red, respectively. The boxed regions in the upper panels are enlarged in the lower panels. Scale bars ⫽ 5 m.
Cell-surface signals and early (5–15 min) intracellular signals of VP22-GFP overlapped only partially with the corresponding signals of CD59. D, HeLa cellstransfected with the pXS-HA-Arf6-Q67L plasmid were allowed to internalize VP22-GFP and anti-CD59 antibody at 37 °C for 60 min, fixed with 4% paraformal-dehyde, and stained with anti-HA antibody. At a late stage (60 min) in Arf6-Q67L-expressing cells, VP22-GFP signals were almost completely co-localized withCD59 signals. Arrows show Arf6-Q67L-induced vacuolar structures. E, HeLa cells treated with 10 mM MCD for 30 min were exposed to 0.5 M VP22-GFP at 37 °C
for 1 h and fixed with 4% paraformaldehyde. MCD appeared to prevent VP22-GFP signals from intracellularly accumulating. MCD-treated cells exhibited a
considerable instability to trypsinization. F, shown are the effects of genistein, cytochalasin D, okadaic acid (OA), vanadate, and PP2 on intracellular VP22-GFPaccumulation. HeLa cells were treated with 100 g/ml genistein, 10 M cytochalasin D, 1 M okadaic acid, 1 mM vanadate, or 10 M PP2 for 30 min and then cells
exposed to 1 M VP22-GFP at 37 °C for 1 h. Measurements from three to six experiments were averaged. Error bars indicate S.D. G, shown are the morphological
changes induced by cytochalasin D treatment. HeLa cells treated with 10 M cytochalasin D for 30 min were exposed to VP22-GFP (0.5 M). VP22-GFP and
phalloidin-labeled rhodamine signals are shown in green and red, respectively. Arrowheads indicate VP22-GFP-accumulated membrane blebs. H, shown is thedrug treatment dependence on cell-surface heparan sulfate and VP22-GFP signals. Except for genistein treatment, no appreciable reduction in cell-surfaceheparan sulfate signals was detected microscopically. In the case of genistein treatment, ⬃60% of the cell-surface heparan sulfate signals were lost. Anti-heparan sulfate (anti-HS) antibody and cell-surface VP22-GFP signals were determined by confocal microscopy (n ⱖ 31). Error bars indicate S.D. I, shown is therelationship between VP22-GFP and caveolin-1 signals. Cells were exposed to 0.5 M VP22-GFP on ice for 1 h, washed, incubated at 37 °C for 15 min, fixed with
4% paraformaldehyde, and stained with anti-caveolin-1 antibody. In the Merged panels, VP22-GFP and caveolin-1 signals are shown in green and red, respec-tively. The boxed regions in the upper panels are enlarged in the lower panels. No overlap between intracellular VP22-GFP and caveolin-1 signals was observed.
Scale bars ⫽ 5 m.
SEPTEMBER 14, 2007 • VOLUME 282 • NUMBER 37
JOURNAL OF BIOLOGICAL CHEMISTRY 27511
Supplemental Material can be found at:http://www.jbc.org/content/suppl/2007/07/24/M703810200.DC1.html
VP22 Internalization via Lipid Raft-mediated Endocytosis
and incubated again at 37 °C for 15min. Hardly any internalized VP22-GFP signals were localized withcaveolin-1 signals (Fig. 5I and sup-plemental Fig. 2B), indicating thatVP22-GFP is internalized inde-pendently of caveolae.
Caveola-mediated endocytosis is
enhanced by okadaic acid, an inhib-itor of several protein serine/threo-nine phosphatases, and vanadate, aninhibitor specific for tyrosine phos-phatases (56). In HeLa cells, okadaicacid treatment enhanced cellularVP22-GFP uptake, but there was noapparent okadaic acid-dependent
stimulation of VP22-GFP internal-ization in CHO-K1 cells (data notshown). Vanadate brought aboutno enhancement of VP22-GFPinternalization but instead signifi-cantly reduced the uptake of
VP22-GFP in either cell type (Fig.
5F). PP2 is an Src kinase-specificinhibitor and prevents caveola-
mediated endocytosis (57). Fig. 5Fshows that VP22-GFP internaliza-tion was completely resistant to PP2treatment. No appreciable changein cell-surface heparan sulfate sig-nals was found upon treatment withokadaic acid, vanadate, or PP2 (Fig.
5H). It follows conclusively fromthese findings that VP22-GFP inter-nalization takes place through acaveola-independent type of lipid
FIGURE 6. Possible roles of small GTPases in VP22-GFP internalization. A, effect of toxin B on intracellular
VP22 accumulation. HeLa cells treated with 0.5 g/ml C. difficile toxin B at 37 °C for 4 h were exposed to 0.5 M
VP22-GFP at 37 °C for 1 h, fixed with 4% paraformaldehyde, and stained with phalloidin-labeled rhodamine.
Rho Family GTPases Are Not
Phalloidin images are those for the bottom of the cells. B, quantitative analysis of toxin B effects. Little effect of
Required for Cellular Internaliza-
toxin B on intracellular VP22 accumulation was observed. Three spectrofluorometric measurements wereaveraged. Error bars indicate S.D. C and D, effect of RhoA RNAi on intracellular VP22 accumulation. RhoA mRNA
tion of VP22-GFP—Small GTPases
was significantly reduced (C), but no reduction in VP22 internalization was evident (D). Firefly luciferase (FL)
are grouped into five families, Ras,
RNAi served as the control. E and F, effect of Cdc42 RNAi on VP22 uptake. Cdc42 mRNA was almost completelyeliminated (E), but little reduction in VP22 internalization was detected (F). G–I, effect of overexpression of
Rho, Rab, Arf, and Ran (58), some of
Rac1-T17N, a dominant-negative form of Rac1. HeLa cells were transfected with pRK5-myc-Rac1-T17N. Twen-
which have been shown recently to
ty-four hours after transfection, cells were exposed to 0.5 M VP22-GFP at 37 °C for 1 h, fixed with 4% paraform-
be differentially involved in lipid
aldehyde, and stained with anti-Myc antibody. In H, cells were also stained with anti-heparan sulfate (anti-HS)antibody. Cells moderately and highly expressing Rac1-T17N are shown in G and H, respectively. Thick arrows
raft-mediated endocytosis (10). Rho
show Rac1-T17N expressing cells, and thin arrows show cells lacking Rac1-T17N expression. Strong internalized
family GTPases include Cdc42,
signals of VP22-GFP and heparan sulfate were found in cells expressing a high level of Rac1-T17N. In these cells,
RhoA, and Rac1. RhoA and Rac1 are
cell-surface signals of VP22 and heparan sulfate were occasionally reduced. Quantitative data are shown in I.
Cell-surface VP22-GFP signals were measured by confocal microscopy (n ⱖ 71). J–L, effect of Arf6 RNAi on
required for lipid raft-mediated
intracellular VP22 and transferrin accumulation. Not only Arf6 mRNA (J) but also VP22 and transferrin internal-
endocytosis of interleukin-2 recep-
ization (K and L) were significantly eliminated subsequent to Arf6 RNAi. HeLa cells transfected with pSUPER-retro-puro-FL or pSUPER-retro-puro-Arf6 were exposed to 0.5 M VP22-GFP and 0.25 M Alexa Fluor 647-
tors (42), whereas Cdc42 is involved
labeled transferrin, fixed with 2% paraformaldehyde, and stained with anti-heparan sulfate antibody.
in that of glycosylphosphatidylino-
Cell-surface heparan sulfate signals appeared to be reduced to some extent in Arf6 knockdown cells.
sitol-anchored proteins (59). C. dif-ficile toxin B specifically inactivates
component of caveolae, and consequently, ligands of caveola-
Rho family GTPases through monoglucosylation (60). To
mediated endocytosis enter caveolin-1-positive subcellular
examine the possible involvement of Rho family GTPases in
compartments (54, 55). To determine the subcellular distribu-
VP22-GFP internalization, HeLa cells were treated with 0.2–1
tion of VP22-GFP at early stages of cellular uptake, HeLa cells
g/ml toxin B, which resulted in typical morphological changes
were incubated in the presence of VP22-GFP at 0 °C, washed,
such as cell rounding and actin filament disruption. As shown
27512 JOURNAL OF BIOLOGICAL CHEMISTRY
VOLUME 282 • NUMBER 37 • SEPTEMBER 14, 2007
Supplemental Material can be found at:http://www.jbc.org/content/suppl/2007/07/24/M703810200.DC1.html
VP22 Internalization via Lipid Raft-mediated Endocytosis
in Fig. 6 (A and B), toxin B treatment brought about significant
At 60 min of internalization, a considerable number of VP22-
changes in cell morphology and the actin cytoskeleton, but
GFP molecules appeared to have moved from early endosomes
there was no appreciable reduction in internalized VP22-GFP
to lysosomes for degradation. Overlapping between VP22-GFP
signals in toxin B-treated cells, suggesting that Rho family
and EEA1 signals at 60 min became less prominent than that at
GTPases are not involved in VP22-GFP internalization.
15 min (Fig. 7, E and I; and supplemental Fig. 3A); instead, some
To further confirm this point, HeLa cells were subjected to
VP22-GFP signals appeared to overlap LAMP1 signals (Fig. 7, F
DNA-mediated RNAi to knock down the genes encoding
and I; and supplemental Fig. 3B), suggesting that VP22-GFP is
RhoA (Fig. 6, C and D) and Cdc42 (E and F). Rac1 activity was
partially transferred to the lysosome at later stages. There was
depleted by overexpressing a dominant-negative form of
no overlap between VP22-GFP and GM130 signals (Fig. 7, G
Rac1 (Rac1-T17N) (Fig. 6, G–I). RhoA and Cdc42 mRNAs
and I; and supplemental Fig. 3C).
were significantly eliminated by RNAi (Fig. 6, C and E), but
Transferrin (internalized through clathrin-mediated endo-
no appreciable reduction in VP22-GFP internalization was
cytosis) accumulates in early endosomes initially and in recy-
detected (D and F). Rac1-T17N expression levels varied
cling endosomes at later stages (63). We thus compared the
depending on the cells. About 70% of the cells expressed
subcellular location of VP22-GFP and transferrin signals at
Rac1-T17N moderately and exhibited a slight increment in
15 and 60 min (Fig. 7, D, H, and I; and supplemental Figs. 2C
internalized VP22-GFP signals without any substantial loss
and 3D). Nearly all VP22-GFP signals were found to have
of surface signals (Fig. 6G), supporting the notion that Rac1
co-localized with transferrin signals at both 15 and 60 min of
is not essential for cellular VP22 internalization. In the
internalization. Thus, the above findings indicate that VP22-
remaining cells, not only cell-surface VP22-GFP signals but
GFP and transferrin, internalized with different mecha-
also cell-surface heparan sulfate signals were frequently
nisms, accumulate in the same early endosomes immediately
observed to be considerably reduced, and instead, the cellu-
following internalization and in recycling endosomes at later
lar internalized signals of both VP22-GFP and heparan sul-
fate were increased (Fig. 6, H and I). We interpreted these
To further confirm the close relationship between internal-
findings as suggesting that high levels of Rac1-T17N expres-
ized VP22-GFP and transferrin in early endosomes, a constitu-
sion might result in rapid cellular uptake of cell-surface com-
tively active Rab5 mutant (Rab5-Q79L) known to stimulate
plexes of heparan sulfate and VP22-GFP. We thus concluded
early endosomal fusion to form ring-shaped large endosomes
that Rho family small GTPases are not essential for normal
(64) was used. In HeLa cells overexpressing wild-type rab5, a
gene encoding wild-type Rab5 tagged with Myc, the cellular
Arf6 is a member of the Arf family and is essential for certain
distribution of VP22-GFP and transferrin was virtually the
lipid raft- or clathrin-mediated endocytosis (10, 61). To show
same as that in non-transfected HeLa cells (data not shown).
Arf6 involvement in VP22-GFP internalization, short hairpin
But in HeLa cells overexpressing Myc-tagged Rab5-Q79L,
RNA specific for arf6 was used to knock down arf6 activity. In
VP22-GFP and transferrin were co-localized with ring-shaped
Fig. 6 (K and L), internalized VP22-GFP and transferrin signals
endosomes, as visualized with anti-Myc antibody (Fig. 7J and
were significantly reduced upon elimination of arf6 activity by
supplemental Fig. 2D). To rule out the possibility that VP22-
RNAi (Fig. 6J). Arf6 is thus assumed to be involved in cellular
GFP enters Rab5-Q79L-positive structures via clathrin-medi-
VP22-GFP internalization. Because cell-surface heparan sulfate
ated endocytosis, the clathrin heavy chain was abolished by
signals were occasionally observed to be reduced moderately in
RNAi (Fig. 7K). In clathrin-depleted cells, VP22-GFP entered
Arf6 knockdown cells (Fig. 6K), Arf6 may be partly involved in
ring-shaped endosomes, and this internalization was inhibited
recruitment of heparan sulfate to the cell surface. Transferrin
by genistein, an inhibitor of lipid raft-mediated endocytosis
internalization was inhibited by arf6 depletion in the HeLa cells
(data not shown). Rab5 and VP22-GFP rings and VP22-GFP
used here; this is inconsistent with previous findings for human
and clathrin rings can be seen to differ slightly in position in Fig.
embryonic kidney 293 and HeLa cells (61, 62) and could suggest
7K, thus possibly reflecting pathway-dependent substructures
differences in cell lines.
of early endosomes.
Co-localization of VP22-GFP and Transferrin in Early
Stabilization of Internalized VP22-GFP Signals in Chloro-
Endosomes—To determine the subcellular localization of inter-
quine-treated Cells—The biological activity of TAT-Cre has
nalized VP22-GFP signals, clarification was sought as to
been shown to be significantly increased by chloroquine
whether VP22-GFP signals are co-localized with cell compart-
treatment (13), possibly suggesting that chloroquine
ment markers. HeLa cells were exposed to VP22-GFP at 0 °C,
enhances endosomal release of these proteins. We thus
and VP22-GFP that was not bound to cells was removed by
examined whether chloroquine treatment induces cytoplas-
washing at 0 °C. VP22-GFP internalization was then examined
mic non-vesicular VP22-GFP signals in HeLa and CHO-K1
by shifting cells to 37 °C. Most, if not all, cytoplasmic VP22-GFP
cells. Cell were treated with 50 M chloroquine, and possible
signals at 15 min of internalization were found to have co-lo-
changes in intracellular VP22-GFP signals were examined
calized with EEA1, an early endosomal marker (Fig. 7A and
spectrofluorometrically and microscopically (Fig. 7, L and
supplemental Fig. 3A), but not with LAMP1, a lysosomal
M). In contrast to our expectations, no apparent increase in
marker (Fig. 7B and supplemental Fig. 3B), or GM130, a Golgi
cytoplasmic non-vesicular VP22-GFP signals was detected
marker (Fig. 7C and supplemental Fig. 3C). Internalized VP22-
even after long chloroquine treatment. Chloroquine treat-
GFP signals are thus initially incorporated into early endo-
ment instead significantly increased intracellular vesicular
somes marked with EEA1.
VP22-GFP signals in both cell lines. We interpret these
SEPTEMBER 14, 2007 • VOLUME 282 • NUMBER 37
JOURNAL OF BIOLOGICAL CHEMISTRY 27513
Supplemental Material can be found at:http://www.jbc.org/content/suppl/2007/07/24/M703810200.DC1.html
VP22 Internalization via Lipid Raft-mediated Endocytosis
results as suggesting that the pre-dicted endosome-cytoplasm transferof VP22-GFP may occur in a mecha-nism different from that of TAT-Cre.
In this work, we have shown that
cell-surface binding and cellularinternalization of VP22 requireinteractions between cell-surfaceheparan sulfate and basic aminoacids in the N-terminal region ofVP22.C1 (C-terminal half of VP22),which includes Arg-164, and thatVP22 is internalized through a lipidraft-mediated endocytic pathway
independent of clathrin, caveolae,and
dependent on dynamin and Arf6.
We have also shown that internal-ized VP22 initially enters earlyendosomes and then moves to ly-
sosomes and possibly recyclingendosomes. Cellular uptake of VP22appears to be unrelated to lipid raft-
mediated endocytosis induced inArf6-Q67L-expressing cells (32).
Differences in Cell-surface Ad-
sorption between VP22-GFP andTAT-PTD—Fig. 2 shows that VP22-GFP bound to the cell surfacemainly via heparan sulfate. The con-tribution of chondroitin sulfate tothis binding is apparently quitesmall, if at all, because virtually noadsorption of VP22-GFP signalscould be detected in pgsD-677, aCHO-K1 cell line capable of pro-ducing 3– 4 times as many chon-droitin sulfate molecules as wild-
FIGURE 7. Incorporation of internalized VP22 signals into early endosomes. A–H, HeLa cells were exposed
type cells without production of
to 0.5 M VP22-GFP and 0.25 M Alexa Fluor 647-labeled transferrin at 0 °C; incubated for 15 min (A–D) or 60
heparan sulfate. The intensity of
min (E–H) at 37 °C; fixed with 4% paraformaldehyde; and stained with anti-EEA1 (A and E), anti-LAMP1 (B and F),and anti-GM130 (C and G) antibodies. Alexa Fluor 647-labeled transferrin signals are shown in D and H. In the
internalized mutant VP22 signals
Merged panels, VP22-GFP is shown in green, whereas EEA1, LAMP1, GM130, and transferrin are shown in red.
was basically in proportion to that of
The boxed regions in the last panels are enlarged in the first three panels. Scale bars ⫽ 10 m. I, shown is the time
signals adsorbed on the cell surface
course of the fraction of intracellular VP22-GFP co-localized with cell compartment markers. HeLa cells wereexposed to 0.5 M VP22-GFP at 0 °C and then incubated for 5, 15, or 60 min at 37 °C. After trypsin treatment at
(Fig. 2, F and G), thus possibly indi-
37 °C for 5 min, cells were stained with anti-EEA1, anti-LAMP1, anti-GM130, and anti-transferrin receptor (TfR)
cating that cell-surface adsorption is
antibodies and visualized by confocal microscopy. Co-localization was determined using the software that
the first rate-limiting step in cellular
came with the confocal microscope (n ⫽ 12). Error bars indicate S.D. J, HeLa cells transfected withpcDNA3-myc-Rab5-Q79L, a plasmid encoding Myc-tagged Rab5-Q79L, were exposed to 0.5 M VP22-GFP and
internalization of VP22-GFP.
0.25 M Alexa Fluor 647-labeled transferrin at 37 °C for 1 h; fixed with 4% paraformaldehyde; and stained with
The role of GAG in cell-surface
anti-Myc antibody. In the Merged panel, VP22-GFP, Myc-Rab5-Q67L, and transferrin are shown in green, red, andblue, respectively. Scale bar ⫽ 10 m. K, HeLa cells transfected with CHC1 siRNA and plasmids expressing
adsorption and internalization of
Myc-Rab5-Q79L were incubated at 37 °C for 1 h with fresh serum-free medium containing 0.5 M VP22-GFP,
the TAT peptide may differ from
fixed with 4% paraformaldehyde, stained with anti-clathrin and anti-Myc antibodies. In the Merged panels,
that of VP22-GFP. The TAT pep-
VP22-GFP and Rab5-Q79L are shown in green and red, respectively. Scale bars ⫽ 5 m. L and M, the effects of
chloroquine treatment on intracellular accumulation of VP22-GFP are shown. In L, CHO-K1 cells treated with 50
tide that enters pgsD-677 cells is
M chloroquine for 30 min were exposed to VP22-GFP (1 M), incubated for 1 or 4 h, and treated with trypsin.
40% as much as that of wild-type
VP22-GFP signals (green) and a differential interference contrast image were merged. In M, HeLa and CHO-K1cells treated with 50
cells, and TAT-Cre internalization
M chloroquine for 30 min were exposed to VP22-GFP (1 M) and incubated for 1 h, and
intracellular accumulation of VP22-GFP signals was determined spectrofluorometrically. Three independent
is significantly inhibited by chon-
measurements were averaged. Error bars indicate S.D.
droitin sulfates B and C (12, 13).
27514 JOURNAL OF BIOLOGICAL CHEMISTRY
VOLUME 282 • NUMBER 37 • SEPTEMBER 14, 2007
Supplemental Material can be found at:http://www.jbc.org/content/suppl/2007/07/24/M703810200.DC1.html
VP22 Internalization via Lipid Raft-mediated Endocytosis
TABLE 1
List of caveola-independent lipid raft-mediated endocytic pathways
⫹, dependent or regulated; ⫺, independent; CHO, Chinese hamster ovary; GPI-APs, glycosylphosphatidylinositol-anchored proteins; M2mAChR, M2 muscarinic acetyl-
choline receptor; CPE, carboxypeptidase E; SV40, simian virus 40; CavDC, caveolin-1-deficient cells; CTB, cholera toxin subunit B; Cav⫺/⫺ MEFs, caveolin-1-null mouse
embryonic fibroblasts; IL-2R, interleukin-2 receptor.
Rho family
Group III
However, Fig. 2B shows that inhibition of VP22-GFP internal-
(32, 42, 57, 59, 69 –71). Our results show that VP22-GFP
ization was hardly inhibited at all by chondroitin sulfate addi-
internalization occurs via lipid raft-mediated endocytosis
tion to the culture medium.
dependent on dynamin and Arf6 but not Rho family GTPases
In TAT-PTD, all 8 basic amino acids are equally required for
such as RhoA, Rac1, and Cdc42. Thus, as summarized in
cellular uptake activity (39). Our mutational analysis of VP22
Table 1, the VP22-GFP endocytic mechanism may differ sig-
(Fig. 2F) indicated that the roles of basic amino acids (23 of 142
nificantly from those previously identified. We presume that
amino acids) of VP22.C1 in cellular VP22-GFP internalization
the endocytic pathway for VP22-GFP internalization may
differ significantly depending on amino acid position. Arg-164
represent the third lipid raft-mediated endocytic pathway
in VP22 is almost indispensable for VP22-GFP internalization
(group III in Table 1).
activity, whereas other amino acids may be only moderately or
VP22.C1 and short peptide-type PTDs may not have the
slightly necessary. Thus, a long PTD such VP22.C1 may differ in
same internalization mechanism. Indeed, short peptide-type
the GAG interaction mode from short peptide-type PTDs such
PTDs such as TAT, the N-terminal peptide of prion protein,
as TAT-PTD. In the former, the amino acid sequence itself
PDX-1, and the octaarginine peptide, in addition to their fusion
appears to be much more important than the presence or
proteins, are internalized via macropinocytosis (13, 14, 72–74).
absence of any positive charge. No or little similarity is found
The TAT peptide itself is internalized via clathrin-dependent
between the VP22 amino acid sequence around position 164
endocytosis (12), and TAT-GFP is internalized via caveola-me-
and the consensus amino acid sequences for heparin/heparan
diated endocytosis in HeLa cells (15, 16).
sulfate-binding domains so far identified (65).
Internalized VP22-GFP was also shown to be initially incor-
Not only specific receptors but also heparan sulfate corecep-
porated into EEA1-positive early endosomes (Fig. 7A) with
tors have been shown to be required for cell-surface binding of
transferrin incorporated via clathrin-mediated endocytosis (D
morphogens such as Hedgehog, bone morphogenetic protein,
and H). Internalized TAT-GFP is not incorporated into EEA1-
Wnt, and fibroblast growth factors (66). Our results in Fig. 2D
positive early endosomes but into caveolin-1-positive struc-
also support the notion that cell-surface VP22-GFP binding
tures (15, 16). This difference in the endocytic mechanism of
requires not only heparan sulfate but also VP22-specific
TAT and VP22 might arise from the difference in their require-
ment for GAGs.
VP22-GFP May Be Internalized through a Unique Endo-
While this manuscript was being revised, Payne et al. (75)
cytic Pathway—Our results show that, in HeLa cells, VP22 is
reported that cell-surface proteoglycans and proteoglycan-
internalized through clathrin/caveola/Rho family GTPase-
binding ligands such as cationic polymers, lipids, and polypep-
independent but dynamin/Arf6-dependent lipid raft-medi-
tides are internalized via clathrin- and caveolin-independent
ated endocytosis. Various clathrin- and caveola-independ-
and flotillin- and dynamin-dependent endocytosis. This system
ent and lipid raft-dependent endocytic pathways have been
is resistant to treatment with cholesterol-binding drugs such as
reported recently (10) and may be classed into two groups
filipin and nystatin and accordingly appears to be independent
based on dynamin dependence (Table 1). In several cell
of lipid rafts (75). In contrast, the clathrin/caveola-independent
types, cellular internalization of glycosylphosphatidylinosi-
but dynamin-dependent endocytic pathway found in VP22
tol-anchored proteins, simian virus 40, and cholera toxin
internalization in this work is mediated by lipid rafts (Fig. 5),
subunit B and Arf6-Q67L-dependent internalization (32) are
suggesting that these two systems are different from each other.
independent of dynamin (group I) (59, 67, 68), but dynamin
In conclusion, we have shown that the mechanism of cellular
is required for cellular internalization of the interleukin-2
uptake for VP22 is significantly different from that for short
receptor and albumin (group II) (42, 57). The former may be
peptide-driven PTDs and that VP22 internalization is carried
regulated by Cdc42 or Arf6, and the latter by RhoA and Rac1
out via a type of lipid raft-mediated endocytosis independent of
SEPTEMBER 14, 2007 • VOLUME 282 • NUMBER 37
JOURNAL OF BIOLOGICAL CHEMISTRY 27515
Supplemental Material can be found at:http://www.jbc.org/content/suppl/2007/07/24/M703810200.DC1.html
VP22 Internalization via Lipid Raft-mediated Endocytosis
clathrin, caveolae, and Rho family GTPases but dependent on
33. Naito, Y., Yamada, T., Ui-Tei, K., Morishita, S., and Saigo, K. (2004) Nu-
dynamin and Arf6.
cleic Acids Res. 32, W124 –W129
34. Ui-Tei, K., Zenno, S., Miyata, Y., and Saigo, K. (2000) FEBS Lett. 479,
Acknowledgments—We thank K. Nakayama, J. Pessin, G. Bokoch,
35. Summerford, C., and Samulski, R. J. (1998) J. Virol. 72, 1438 –1445
and J. G. Donaldson for plasmids and K. Ui-Tei for helpful discussion.
36. Tang, Y., and DeFranco, D. B. (1996) Mol. Cell. Biol. 16, 1989 –2001
37. Esko, J. D., Stewart, T. E., and Taylor, W. H. (1985) Proc. Natl. Acad. Sci.
U. S. A. 82, 3197–3201
38. Lidholt, K., Weinke, J. L., Kiser, C. S., Lugemwa, F. N., Bame, K. J., Cheifetz,
1. Wadia, J. S., and Dowdy, S. F. (2002) Curr. Opin. Biotechnol. 13, 52–56
S., Massague, J., Lindahl, U., and Esko, J. D. (1992) Proc. Natl. Acad. Sci.
2. Lindsay, M. A. (2002) Curr. Opin. Pharmacol. 2, 587–594
U. S. A. 89, 2267–2271
3. Green, M., and Loewenstein, P. (1988) Cell 55, 1179 –1188
39. Wender, P. A., Mitchell, D. J., Pattabiraman, K., Pelkey, E. T., Steinman, L.,
4. Frankel, A., and Pado, C. (1988) Cell 55, 1189 –1193
and Rothbard, J. B. (2000) Proc. Natl. Acad. Sci. U. S. A. 97, 13003–13008
5. Vive s, E., Brodin, P., and Lebleu, B. (1997) J. Biol. Chem. 272,
40. van der Bliek, A. M., Redelmeier, T. E., Damke, H., Tisdale, E. J., Meyer-
owitz, E. M., and Schmid, S. L. (1993) J. Cell Biol. 122, 553–563
6. Derossi, D., Joliot, A. H., Chassaing, G., and Prochiantz, A. (1994) J. Biol.
41. Oh, P., McIntosh, D. P., and Schnitzer, J. E. (1998) J. Cell Biol. 141,
Chem. 269, 10444 –10450
7. Richard, J. P., Melikov, K., Vive s, E., Ramos, C., Verbeure, B., Gait, M. J.,
42. Lamaze, C., Dujeancourt, A., Baba, T., Lo, C. G., Benmerah, A., and Dau-
Chernomordik, L. V., and Lebleu, B. (2003) J. Biol. Chem. 278, 585–590
try-Varsat, A. (2001) Mol. Cell 7, 661– 671
8. Lundberg, M., Wikstrom, S., and Johansson, M. (2003) Mol. Ther. 8,
43. Sauvonnet, N., Dujeancourt, A., and Dautry-Varsat, A. (2005) J. Cell Biol.
9. Conner, S. D., and Schmid, S. L. (2003) Nature 422, 37– 44
44. Wang, L. H., Rothberg, K. G., and Anderson, R. G. (1993) J. Cell Biol. 123,
10. Kirkham, M., and Parton, R. G. (2005) Biochim. Biophys. Acta 1745,
45. Motley, A., Bright, N. A., Seaman, M. N., Margaret S., and Robinson, M. S.
11. Tyagi, M., Rusnati, M., Presta, M., and Giacca, M. (2001) J. Biol. Chem.
(2003) J. Cell Biol. 162, 909 –918
276, 3254 –3261
46. Hinrichsen, L., Harborth, J., Andrees, L., Weber, K., and Ungewickell, E. J.
12. Richard, J. P., Melikov, K., Brooks, H., Prevot, P., Lebleu, B., and Cherno-
(2003) J. Biol. Chem. 278, 45160 – 45170
mordik, L. V. (2005) J. Biol. Chem. 280, 15300 –15306
47. Jackson, A. L., and Linsley, P. S. (2004) Trends Genet. 20, 521–524
13. Wadia, J. S., Stan, R. V., and Dowdy, S. F. (2004) Nat. Med. 10, 310 –315
48. Simons, K., and Ikonen, E. (1997) Nature 387, 569 –572
14. Kaplan, I. M., Wadia, J. S., and Dowdy, S. F. (2005) J. Controlled Release
49. Martinho, R. G., Castel, S., Urena, J., Fernandez-Borja, M., Makiya, R.,
Olivecrona, G., Reina, M., Alonso, A., and Vilaro, S. (1996) Mol. Biol. Cell
15. Fittipaldi, A., Ferrari, A., Zoppe, M., Arcangeli, C., Pellegrini, V., Beltram,
F., and Giacca, M. (2003) J. Biol. Chem. 278, 34141–34149
50. Nabi, I. R., and Le, P. U. (2003) J. Cell Biol. 161, 673– 677
16. Ferrari, A., Pellegrini, V., Arcangeli, C., Fittipaldi, A., Giacca, M., and Bel-
51. Pelkmans, L., and Helenius, A. (2002) Traffic 3, 311–320
tram, F. (2003) Mol. Ther. 8, 284 –294
52. Robert, G. P., and Richards, A. A. (2003) Traffic 4, 724 –738
17. Jones, S. W., Christison, R., Bundell, K., Voyce, C. J., Brockbank, S. M.,
53. Gauthier, N. C., Ricci, V., Gounon, P., Doye, A., Tauc, M., Poujeol, P., and
Newham, P., and Lindsay, M. A. (2005) Br. J. Pharmacol. 145,
Boquet, P. (2004) J. Biol. Chem. 279, 9481–9489
54. Pelkmans, L., Kartenbeck, J., and Helenius, A. (2001) Nat. Cell Biol. 3,
18. Vendeville, A., Rayne, F., Bonhoure, A., Bettache, N., Montcourrier, P.,
and Beaumelle, B. (2004) Mol. Biol. Cell 15, 2347–2360
55. Nichols, B. J. (2002) Nat. Cell Biol. 4, 374 –378
19. Potocky, T. B., Menon, A. K., and Gellman, S. H. (2003) J. Biol. Chem. 278,
56. Pelkmans, L., Puntener, D., and Helenius, A. (2002) Science 296, 535–539
57. Cheng, Z. J., Singh, R. D., Sharma, D. K., Holicky, E. L., Hanada, K., Marks,
20. Fischer, R., Kohler, K., Fotin-Mleczek, M., and Brock, R. (2004) J. Biol.
D. L., and Pagano, R. E. (2006) Mol. Biol. Cell 17, 3197–3210
Chem. 279, 12625–12635
58. Wennerberg, K., Rossman, K. L., and Der, C. J. (2005) J. Cell Sci. 118,
21. Elliott, G. D., and Meredith, D. M. (1992) J. Gen. Virol. 73, 723–726
22. Elliott, G., and O'Hare, P. (1997) Cell 88, 223–233
59. Sabharanjak, S., Sharma, P., Parton, R. G., and Mayor, S. (2002) Dev. Cell 2,
23. Kueltzo, L. A., Normand, N., O'Hare, P., and Middaugh, C. R. (2000) J. Biol.
Chem. 275, 33213–33221
60. Aktories, K., and Just, I. (1995) Trends Cell Biol. 5, 441– 443
24. Normand, N., van Leeuwen, H., and O'Hare, P. (2001) J. Biol. Chem. 276,
61. Houndolo, T., Boulay, P. L., and Claing, A. (2005) J. Biol. Chem. 280,
25. Stroh, C., Held, J., Samraj, A. K., and Schulze-Osthoff, K. (2003) Oncogene
62. Hashimoto, S., Hashimoto, A., Yamada, A., Kojima, C., Yamamoto, H.,
Tsutsumi, T., Higashi, M., Mizoguchi, A., Yagi, R., and Sabe, H. (2004)
26. Brewis, N. D., Phelan, A., Normand, N., Choolun, E., and O'Hare, P. (2003)
J. Biol. Chem. 279, 37677–37684
Mol. Ther. 7, 262–270
63. Ghosh, R. N., and Maxfield, F. R. (1995) J. Cell Biol. 128, 549 –561
27. Zavaglia, D., Normand, N., Brewis, N., O'Hare, P., Favrot, M. C., and Coll,
64. Stenmark, H., Parton, R. G., Steele-Mortimer, O., Lutcke, A., Gruenberg,
J. (2003) Mol. Ther. 8, 840 – 845
J., and Zerial, M. (1994) EMBO J. 13, 1287–1296
28. Boenicke, L., Chu, K., Pauls, R., Tams, C., Kruse, M. L., Kurdow, R.,
65. Capila, I., and Linhardt, R. J. (2002) Angew. Chem. Int. Ed. Engl. 41,
Schniewind, B., Bohle, A., Kremer, B., and Kalthoff, H. (2003) J. Mol. Med.
66. Filmus, J., and Selleck, S. B. (2001) J. Clin. Investig. 108, 497–501
29. Narita, K., Choudhury, A., Dobrenis, K., Sharma, D. K., Holicky, E. L.,
67. Damm, E. M., Pelkmans, L., Kartenbeck, J., Mezzacasa, A., Kurzchalia, T.,
Marks, D. L., Walkley, S. U., and Pagano, R. E. (2005) FASEB J. 19,
and Helenius, A. (2005) J. Cell Biol. 168, 477– 488
68. Kirkham, M., Fujita, A., Chadda, R., Nixon, S. J., Kurzchalia, T. V., Sharma,
30. Kasai, K., Shin, H. W., Shinotsuka, C., Murakami, K., and Nakayama, K.
D. K., Pagano, R. E., Hancock, J. F., Mayor, S., and Parton R. G. (2005) J. Cell
(1999) J. Biochem. (Tokyo) 125, 780 –789
Biol. 168, 465– 476
31. Steiner, P., Floyd Sarria, J. C., Glauser, L., Magnin, S., Catsicas, S., and
69. Gauthier, N. C., Monzo, P., Kaddai, V., Doye, A., Ricci, V., and Boquet, P.
Hirling, H. (2002) J. Cell Biol. 157, 1197–1209
(2005) Mol. Biol. Cell 16, 4852– 4866
32. Naslavsky, N., Weigert, R., and Donaldson, J. G. (2004) Mol. Biol. Cell 15,
70. Delaney, K. A., Murph, M. M., Brown, L. M., and Radhakrishna, H. (2002)
J. Biol. Chem. 277, 33439 –33446
27516 JOURNAL OF BIOLOGICAL CHEMISTRY
VOLUME 282 • NUMBER 37 • SEPTEMBER 14, 2007
Supplemental Material can be found at:http://www.jbc.org/content/suppl/2007/07/24/M703810200.DC1.html
VP22 Internalization via Lipid Raft-mediated Endocytosis
71. Arnaoutova, I., Jackson, C. L., Al-Awar, O. S., Donaldson, J. G., and Loh,
S., Masui, Y., Futaki, S., and Tanaka, K. (2005) Cell Transplant. 14,
Y. P. (2003) Mol. Biol. Cell 14, 4448 – 4457
72. Magzoub, M., Sandgren, S., Lundberg, P., Oglecka, K., Lilja, J., Wittrup, A.,
74. Nakase, I., Niwa, M., Takeuchi, T., Sonomura, K., Kawabata, N., Koike, Y.,
Go¨ran Eriksson, L. E., Langel, U., Belting, M., and Gra¨slund, A. (2006)
Takehashi, M., Tanaka, S., Ueda, K., Simpson, J. C., Jones, A. T., Sugiura,
Biochem. Biophys. Res. Commun. 348, 379 –385
Y., and Futaki, S. (2004) Mol. Ther. 10, 1011–1022
73. Noguchi, H., Matsumoto, S., Okitsu, T., Iwanaga, Y., Yonekawa, Y.,
75. Payne, C. K., Jones, S. A., Chen, C., and Zhuang, X. (2007) Traffic 8,
Nagata, H., Matsushita, M., Wei, F. Y., Matsui, H., Minami, K., Seino,
SEPTEMBER 14, 2007 • VOLUME 282 • NUMBER 37
JOURNAL OF BIOLOGICAL CHEMISTRY 27517
Source: http://ui-tei.rnai.jp/assets/files/pdf/Cellular%20Internalization.pdf
caresuper.com.au
insurance application form 1. Your personal details You can complete the CareSuper member number Date of birth (DD/MM/YYYY) insurance application process online via the Insurance section of MemberOnline at caresuper.com.au Instructions To apply to change your State/Territory Postcode occupational category, complete sections 1, 2 and 9e, and ensure you read Telephone (home)
kznathletics.co.za
ATHLETICS OMNIBUS - ILLEGAL SUBSTANCES IN SPORT From the Boland Athletics website: www.bolandathletics.com THE USE OF PROHIBITED SUBSTANCES IN SPORT A PROHIBITED CLASSES OF SUBSTANCES The International Association of Athletics Federations (IAAF) rule 144 states clearly that any method used to enhance performance artificially is illegal.