Nutrologia.med.br
The Effects of Serum Testosterone, Estradiol, and SexHormone Binding Globulin Levels on Fracture Risk in
Older Men
Erin S. LeBlanc, Carrie M. Nielson, Lynn M. Marshall, Jodi A. Lapidus,Elizabeth Barrett-Connor, Kristine E. Ensrud, Andrew R. Hoffman, Gail Laughlin,Claes Ohlsson, and Eric S. Orwoll, for the Osteoporotic Fractures in Men StudyGroup Bone and Mineral Unit (E.S.L., C.M.N., L.M.M., J.A.L., E.S.O.), Oregon Health and Science University,Portland, Oregon 97239; Department of Family and Preventive Medicine (E.B.-C., G.L.), University ofCalifornia, San Diego, San Diego, California 92093; Departments of Medicine and Epidemiology &Community Health (K.E.E.), University of Minnesota and Department of Medicine (K.E.E.), VeteransAffairs Medical Center, Minneapolis, Minnesota 55417; Department of Medicine (A.R.H.), StanfordUniversity, Palo Alto, California 94305; and Department of Internal Medicine (C.O.), Center for BoneResearch at the Sahlgrenska Academy, SE-416 85 Go¨teborg, Sweden Context: The relationship between sex steroids and fracture is poorly understood.
Objective: The objective of the study was to examine associations between nonvertebral fracture
risk and bioavailable estradiol (bioE2), bioavailable testosterone (bioT), and SHBG.
Design: This was a case-cohort study.
Setting: The Osteoporotic Fractures in Men Study (MrOS) was conducted in a prospective U.S.
cohort in 5995 community-dwelling men 65 yr old or older.
Participants: Participants included a subcohort of 1436 randomly chosen white men plus all 446
minorities and all those with incident hip and other nonvertebral fractures.
Main Outcome Measures: Baseline testosterone and estradiol were measured by mass spectrom-
etry (MS) and SHBG by RIA.
Results: Men with the lowest bioE2 (⬍11.4 pg/ml) or highest SHBG (⬎59.1 nM) had greater risk of
all nonvertebral fractures [adjusted hazard ratio (HR) [95% confidence interval]: 1.5 (1.2–1.9) and
1.4 (1.1–21.8), respectively]. Men with the lowest bioT (⬍163.5 ng/dl) had no increased fracture risk
after adjustment for bioE2 [adjusted HR 1.16 (0.90 –1.49)]. A significant interaction between SHBG
and bioT (P ⫽ 0.03) resulted in men with low bioT and high SHBG having higher fracture risk [HR
2.1 (1.4 –3.2)]. Men with low bioE2, low bioT, and high SHBG were at highest risk [HR 3.4 (2.2–5.3)].
Conclusions: Older men with low bioE2 or high SHBG levels are at increased risk of nonvertebral
fracture. When SHBG levels are high, men with low bioT levels have higher risk. The strongest
association occurred when all measures were considered in combination. (J Clin Endocrinol Metab
94: 3337–3346, 2009)
Ithasbeenspeculatedthatsexsteroidscontributetofrac- gens and estrogens have in vitro and in vivo bone effects
ture risk in older men (1). With aging, sex steroid con- and trophic effects on skeletal development (6). Estradiol centrations decline (2, 3), fracture rate increases (4), and has been consistently associated with skeletal character- testosterone therapy improves bone density (5). Andro- istics (6 –11), but whether testosterone has independent ISSN Print 0021-972X ISSN Online 1945-7197 Abbreviations: bioE2, Bioavailable estradiol; bioT, bioavailable testosterone; BMD, bone Printed in U.S.A.
mineral density; BMI, body mass index; CI, confidence interval; CV, coefficient of variation; Copyright 2009 by The Endocrine Society HR, hazard ratio; MrOS, Osteoporotic Fractures in Men Study.
doi: 10.1210/jc.2009-0206 Received February 13, 2009. Accepted June 25, 2009.
First Published Online July 7, 2009 J Clin Endocrinol Metab, September 2009, 94(9):3337–3346
LeBlanc et al.
Sex Steroids and Fracture in Men
J Clin Endocrinol Metab, September 2009, 94(9):3337–3346
effects on bone density, structure, or biochemical indi-ces is uncertain (12). Testosterone may affect variousextraskeletal functions relevant to fracture, includingmuscle strength, physical activity, cognition, and fallrate (13–18).
High SHBG has been independently associated with
fracture risk (19 –25). By binding to testosterone andestradiol, SHBG reduces circulating sex steroid concen-trations and thereby their cellular actions. SHBG mayhave independent effects via a receptor mediated mech-anism or affect sex steroid interaction with cellular re-ceptors (26 –28).
Although several publications suggest lower estra-
diol and/or testosterone or higher SHBG are linked tohigher fracture rates (11, 19 –21, 29), few studies haveadequate power to assess independent and/or interde-pendent effects of estradiol, testosterone, and SHBG.
Most previous studies measured sex steroids using RIAtechniques, which are susceptible to artifact, particu-larly at low concentrations (30, 31).
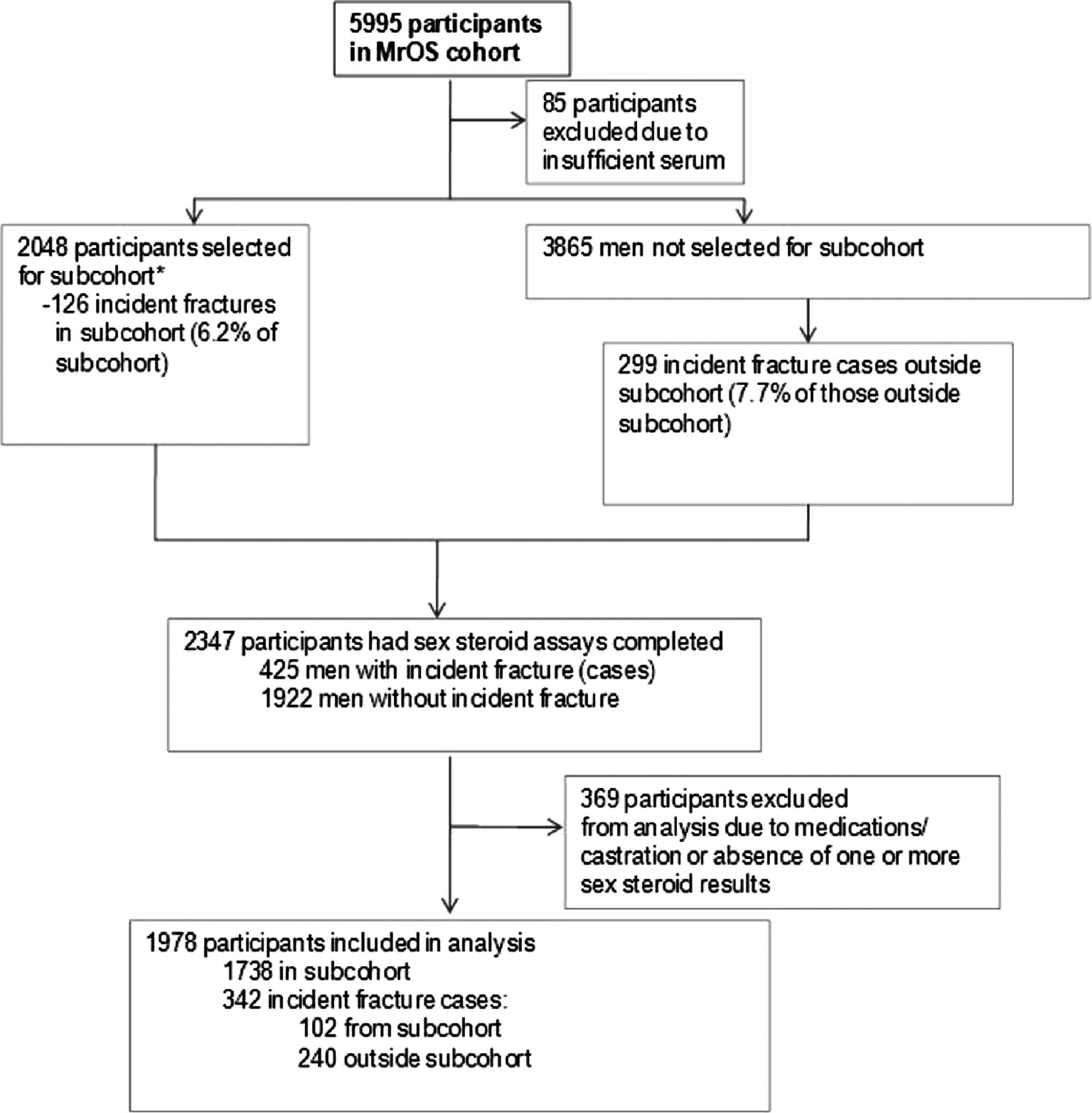
FIG. 1. Case-cohort design for the MrOS sex steroids and fracture
We report associations between fracture risk and sex
study. *, Subcohort consisted of 1436 randomly selected non-Hispanic
steroids in a large cohort of older men. Sex steroid levels
white men and all 446 minority men. Weighting was used in analysesto account for stratified sampling by race.
were measured using liquid chromatography/massspectrometry, a method with high accuracy (32, 33). We
subcohort and 342 incident fracture cases (102 from subcohort;
examined interactions between sex steroids, fracture
240 outside subcohort).
risk, and other variables including bone mineral density(BMD), age, body composition, physical activity, and
physical performance. We assessed the SHBG-fracture
Race/ethnicity, education level, smoking and alcohol con-
sumption, occurrence of fracture after age 50 yr, medical history,
association, both independently and in combination
and previous 12-month fall occurrence were determined by ques-
with sex steroids.
tionnaire at baseline. Current medications were recorded. Phys-ical activity was assessed with the Physical Activity Score for theElderly (36). Height (centimeters) and weight (kilograms) weremeasured using standard protocols. Grip strength (kilograms),
Subjects and Methods
lower extremity power, time to complete a narrow walk (6 m ⫻20 cm), and ability to rise from a chair without arms were as-
sessed (34).
The Osteoporotic Fractures in Men Study (MrOS) study en-
rolled 5995 participants from March 2000 through April 2002
Sex steroid measurements
as previously described (34, 35). Community-based recruitment
Baseline fasting morning blood was collected. Serum was pre-
occurred at six U.S. academic medical centers in Birmingham,
pared immediately after phlebotomy and stored at ⫺70 C. Total
AL; Minneapolis, MN; Palo Alto, CA; Pittsburgh, PA; Portland,
serum testosterone and estradiol were measured using a com-
OR; and San Diego, CA. Eligible participants were at least 65 yr
bined gas chromatographic-negative ionization tandem mass
old, could walk without assistance, and had not had bilateral hip
spectrometry and liquid chromatographic electrospray tandem
replacement surgery. The institutional review board at each cen-
mass spectrometry bioanalytical method (Taylor Technology,
ter approved the study protocol. All participants gave written
Princeton, NJ). A 1/(concentration)2 weighted least squares re-
informed consent. We used a case-cohort design: a random sub-
gression procedure was used to fit a linear function to the cali-
sample of the original cohort (subcohort) was selected indepen-
bration data. The lower limit of detection for estradiol is 0.625
dently of fracture cases, and all cases outside the subsample were
pg/ml (2.29 pmol/liter), and for testosterone is 25.0 pg/ml (0.09
selected (Fig. 1). We selected 2048 men for steroid measurements
nmol/liter). Duplicate aliquots from each participant's serum
(subcohort). A total of 1436 were randomly chosen plus all 446
were assayed and results averaged. Testosterone intraassay co-
minorities were included. They were followed for 4.7 (⫾.9) yr.
efficient of variation (CV) was 2.5% and interassay CV, 6.0%;
Measures were also obtained in men (n ⫽ 3865) who experienced
the estradiol intraassay CV was 6.4% and interassay CV, 10.1%.
an incident nonvertebral fracture between enrollment and July
Serum SHBG concentrations were measured using an Immulite
2006. Therefore, fracture cases could arise from either the sub-
analyzer with chemiluminescent substrate (Diagnostic Products
cohort (n ⫽ 126) or the remainder of the cohort (n ⫽ 299). After
Corp., Los Angeles, CA). The standard curve ranged from 0.2 to
exclusions, the final study population included 1738 men in the
180 nm/liter. The SHBG intraassay CV was 4.4% and interassay
J Clin Endocrinol Metab, September 2009, 94(9):3337–3346
CV, 6.0%. Albumin values for free hormone calculations were
(40) and were considered statistically significant if P ⬍ 0.10. We
obtained from baseline serum using routine colorimetric
then categorized men into eight mutually exclusive categories.
methods (interassay CV 2.0%). Calculation of bioavailable
The reference category (lowest risk) contained men with bioT
fractions of testosterone and estradiol was by the method of
and bioE2 in the highest three quartiles and SHBG in the lowest
Sodergard et al. (37). Using this method, the Spearman cor-
three quartiles. The eighth category (highest risk) contained men
relation coefficient for bioavailable testosterone and free tes-
with bioT and bioE2 in the lowest quartile and SHBG in the
tosterone was 0.98 and for bioavailable estradiol and free
highest quartile. Each intermediate category contained men who
estradiol was 0.98, both P ⬍ 0.0001.
were in one or more high-risk quartiles of bioT, bioE2, or SHBG.
All Cox proportional hazard models were fit using the
weighting method of Barlow et al. (41) for case-cohort analysis.
Areal proximal femur BMD was measured using dual-energy
Age, race, and body mass index (BMI) were included as covari-
x-ray absorptiometry (QDR 4500W; Hologic Inc., Bedford,
ates in all models. Additional potential confounders were added,
MA). Participants were scanned according to standardized pro-
and if addition changed the HR for the sex steroid variable by
cedures and scanners were calibrated at baseline. Whole body,
more than 10%, it was retained in the model. Primary analyses
spine, hip, and linearity phantoms were measured at all sites at
were of each sex steroid individually. Subsequently models were
baseline, and spine and hip phantoms were scanned throughout
adjusted for other sex steroids. For example, the model evalu-
the study to monitor longitudinal changes. Daily quality control
ating bioE2 was also adjusted for the dichotomous bioT and
scans showed no shifts in scanner performance at any site during
SHBG variables to determine whether this altered the HR for
To estimate the proportion of fracture cases that would be
attributable to low bioE2, low bioT, and high SHBG, we con-
Ascertainment of incident fractures
ducted an exploratory attributable fraction analysis. The av-
We contacted 99% of participants every 4 months by mail or
erage attributable fraction method (42) was used to obtain
telephone to ask about recent fractures. All reported nonspine
attributable fraction estimates for each sex steroid and SHBG
fractures were adjudicated by physician review of radiology re-
and adjust for the other sex steroid/SHBG measures and for
ports or x-rays if radiology reports were unavailable. Fracture
age, BMI, and BMD. To conduct this exploration with readily
follow-up was 99%. Using a group of investigators, fractures
available statistical code (43), we assumed a simple case-con-
were adjudicated as traumatic if circumstances leading to the
trol design and estimated odds ratios using multivariable lo-
fracture would likely have resulted in a fracture in a normal
gistic regression.
To determine the robustness of our findings, we performed
sensitivity analyses. To evaluate whether models were robust to
potentially influential observations, we calculated Df for each
Cox proportional hazards models, with weighting to accom-
of the sex steroid variables in the final models, with and without
modate the stratified sampling and case-cohort design, were used
interaction terms. Using a cutoff of the absolute value of 2/冑n, no
to evaluate associations between sex steroids and time to incident
points were considered influential. However, plots of each Df
by identification number allowed us to identify those observa-
Three methods were used to evaluate associations between
tions with relatively more influence than others. When these
sex steroids and time to first fracture. We first created quartiles
were excluded (n ⫽ 3 for full model without interaction term, n ⫽
of sex steroid variables based on distributions in the subcohort.
15 for full model with interaction term), there were no changes
Because men in second, third, and fourth quartiles had similar
in tests of the null hypothesis (i.e. no term gained or lost statistical
risks of fracture, we created dichotomous variables; for testos-
significance), and only the adjusted HR for bioE2 was attenuated
terone and estradiol, the lowest quartile was compared with the
(by 0.1%). The HRs for other terms were unchanged or strength-
other three quartiles; for SHBG, the highest quartile was com-
ened by the exclusion of observations with relatively larger ab-
pared with the lowest three quartiles. Second, we used restricted
solute values of Df.
cubic spline Cox proportional hazard models to examine sexsteroid variables as continuous and to test whether associationswith incident fracture were nonlinear (38). Third, we performed
exploratory cut point analysis. We dichotomized sex steroids atvarious quantiles using log likelihoods of Cox proportional haz-
Most nonvertebral fractures were judged as nontrau-
ard models. The cut point at which the sex steroid variable was
matic (nontraumatic n ⫽ 280, traumatic n ⫽ 62). There
dichotomized to produce the highest profile log likelihood was
were few traumatic hip fractures (n ⫽ 2), and their ex-
considered the best value for further dichotomizing (39). The
clusion did not affect analyses. The subcohort and frac-
cubic spline and cut point analyses supported use of the firstquartile as a cut point.
ture case characteristics are shown in Table 1. Corre-
We evaluated interactions among bioavailable testosterone
lations between serum levels of sex steroids and SHBG
(bioT), bioavailable estradiol (bioE2), and SHBG. We stratified
[bioE2 and SHBG: r ⫽ ⫺0.13 (P ⬍ 0.0001), bioT and
each dichotomous sex steroid variable (dichotomized at lowest
SHBG: r ⫽ 0.27 (P ⬍ 0.0001)] and between bioE2 and
quartile for bioE2 and bioT and highest quartile for SHBG) and
bioT [r ⫽ 0.37 (P ⬍ 0.0001)] were moderate. Age was
evaluated adjusted hazard ratios (HRs) for remaining sex steroidvariables in each stratum. For example, we tested the association
negatively associated with bioT and bioE2 (r ⫽ ⫺0.19
between bioT and fracture in each stratum of SHBG. Additive
to ⫺0.09) and positively associated with SHBG (r ⫽
interactions were tested in Cox proportional hazards models
0.24) (P ⬍ 0.0001). BMI was negatively associated with
LeBlanc et al.
Sex Steroids and Fracture in Men
J Clin Endocrinol Metab, September 2009, 94(9):3337–3346
TABLE 1. Selected characteristics of men in the MrOS sex steroid case-cohort study
Subcohort (n ⴝ 1738)a fracture cases (n ⴝ 1636)
cases (n ⴝ 342)b
Mean ⴞ SD or %
Mean ⴞ SD or %
Mean ⴞ SD or %
Self-reported health
Fair/poor/very poor
Cigarette smoking
Current alcohol consumption
Greater than zero and less than seven drinks
Seven or more drinks per week
Physical performance
Narrow walk (m/sec)
Leg power (100 W)
History of falls reported at baseline
Previous nontrauma fracture after age 50 yr
Total testosterone (ng/dl)
Total estradiol (pg/ml)
BioT (ng/dl)c
BioE2 (pg/ml)c
a Subcohort consisted of 1436 randomly selected non-Hispanic white men and all 446 minority men. It includes 102 incident fracture cases (Figure1). Minorities were oversampled in the subcohort; b fracture cases include 102 incident fracture cases inside the subcohort and 240 incidentfracture cases outside the cohort (Figure 1). Of the nonvertebral fractures, 74 (21.6%) were hip fractures; c to convert bioE2 to picomoles per liter,the conversion factor is 3.671; to convert bioT to nanomoles per liter, the conversion factor is 0.0347.
bioT and SHBG (r ⫽ ⫺0.31 to ⫺0.30) and positively
BMI, the HR for all nonspine fracture in those in the lowest
associated with bioE2 (r ⫽ 0.17) (P ⬍ 0.0001). Weight
bioE2 quartile vs. the highest three quartiles was 1.48
decreased by 0.36% per year during follow-up. Corre-
[95% confidence interval (CI) 1.18 –1.86; Table 2 and Fig.
lations between bioT, bioE2, and SHBG and BMD were
2A]. The association was similar after adjustment for bioT
between ⫺0.05 and ⫺0.2 (P ⬍ 0.0001).
and SHBG but was somewhat attenuated after adjustmentfor total hip BMD (HR 1.29; 95% CI 1.01–1.64). A sim-
Fracture risk and sex steroids
ilar association was present between bioE2 and hip frac-
Men with lower levels of bioE2 were at higher risk of
ture risk (HR 1.57; 95% CI 0.95–2.59; Table 3). Total
nonvertebral fracture. After adjustment for age, race, and
estradiol was not significantly associated with nonverte-
TABLE 2. Hazard ratios (95% CI) for association between nonvertebral fractures and sex steroids
1.49 (1.19 –1.87)
1.39 (1.10 –1.75)
1.63 (1.30 –2.04)
Adjusted for age, race, BMI
1.48 (1.18 –1.86)
1.28 (1.00 –1.64)
1.44 (1.14 –1.82)
Adjusted for bioE2c
1.16 (0.90 –1.49)
1.42 (1.12–1.80)
Adjusted for bioTc
1.42 (1.12–1.80)
1.48 (1.17–1.88)
Adjusted for SHBGc
1.46 (1.16 –1.83)
1.33 (1.04 –1.70)
Full model including bioE2, bioT, and SHBGc
1.39 (1.09 –1.76)
1.20 (0.93–1.56)
1.45 (1.14 –1.84)
Full model additionally adjusted for BMDc
1.29 (1.01–1.64)
1.24 (0.96 –1.59)
1.36 (1.07–1.72)
a HR is for lowest quartile vs. highest three; for bioE2 lowest quartile was less than 11.4 pg/ml (⬍41.8 pmol/liter); for bioT lowest quartile was lessthan 163.5 ng/dl (⬍5.67 nmol/liter); b HR is for highest quartile (SHBG ⱖ59.1 nM) vs. lowest three; c also adjusted for age, race, and BMI; BMDrefers to total hip BMD.
J Clin Endocrinol Metab, September 2009, 94(9):3337–3346
no longer significant after adjustment for bioE2 (HR 1.16;
95% CI 0.90 –1.49). When only nontraumatic fractureswere considered, the association between bioT and frac-ture risk was stronger (HR 1.45; 95% CI 1.12–1.89) and
remained significant after adjustment for bioE2 (HR 1.31;
95% CI 1.00 –1.72). Inclusion of BMD in the model did
not significantly affect the association, regardless oftrauma status. The HRs for the relationship between bioTand hip fracture risk were similar (Table 3). Total testos-terone levels were not associated with nonvertebral (HR1.02; 95% CI 0.79 –1.32) or hip fracture risk (HR 0.93;
< 11.4 11.4-14.0 14.1-17.0 > 17.1
95% CI 0.51–1.71).
Quartile of bioavailable estradiol (pg/ml)
Fracture risk and SHBG
After adjustment for age, race, and BMI, men with the
highest quartile of SHBG were at increased risk of non-vertebral fracture compared with those in the lowest three
quartiles (HR 1.44; 95% CI 1.14 –1.82; Table 2 and Fig.
2C). The association remained consistent after adjustment
for sex steroids but was slightly attenuated after adjust-ment for BMD. Associations between SHBG level andfracture risk were slightly stronger but not substantivelyaltered when only nontraumatic fractures were considered(HR 1.57; 95% CI 1.22–2.03). Hip fracture risk was ap-
< 163.5 163.5-202.3 202.4-242.8
proximately doubled in men with high SHBG (HR 2.17;
Quartile of bioavailable testosterone (ng/dl)
95% CI 1.31–3.59; Table 3) and was not influenced by
further adjustment for sex steroids or BMD.
Consideration of covariates
The associations between fracture risk and sex ste-
roids and SHBG were not substantively altered by se-quential adjustment for other potential confounders,including physical activity, physical performance, andprevious falls. Limiting analyses to non-Hispanic whiteparticipants and excluding hip fractures did not alterthe findings.
< 35.3 35.3-45.9 46.0-59.0 > 59.1
Quartile of SHBG (nM)
FIG. 2. HRs and 95% CIs for risk of nonvertebral fractures by quartiles
of sex steroids (adjusted for age, race, BMI). A, Bioavailable estradiol.
Spline analyses showed a nonlinear association be-
B, Bioavailable testosterone. C, SHBG. To convert bioavailable estradiol
tween serum bioE2 and nonvertebral fracture (P for non-
to picomoles per liter, the conversion factor is 3.671; to convertbioavailable testosterone to nanomoles per liter, the conversion factor
linearity ⫽ 0.045; Fig. 3A). Log likelihood cut point anal-
ysis showed that dichotomizing bioE2 at 12.5 pg/ml (45.9pmol/liter) maximized model fit for nonvertebral frac-
bral (HR 1.09; 95% CI 0.86 –1.39) or hip fracture risk
tures. This threshold concentration was similar to that
(HR 1.52; 95% CI 0.91–2.52). These associations were
associated with increased fracture risk in the lowest quar-
essentially unchanged when only nontraumatic fractures
tile of bioE2 [⬍11.4 pg/ml (41.8 pmol/liter)]. Spline anal-
were considered.
ysis did not reveal nonlinearity in the associations between
After adjustment for age, race, and BMI, men with bioT
fracture risk and bioT or SHBG (Fig. 3, B and C).
in the lowest quartile had a higher risk of nonvertebralfracture than those in the highest three quartiles (HR 1.28;
Interaction between bioT, SHBG, and fracture risk
95% CI 1.00 –1.64; Table 2 and Fig. 2B). The association
We observed a significant additive interaction between
was slightly stronger after adjustment for SHBG but was
bioT and SHBG (P ⫽ 0.03). Nonvertebral fracture risk for
LeBlanc et al.
Sex Steroids and Fracture in Men
J Clin Endocrinol Metab, September 2009, 94(9):3337–3346
TABLE 3. Hazard ratios (95% CI) for association between hip fractures and sex steroids
1.56 (0.96 –2.54)
1.74 (1.05–2.86)
3.53 (2.20 –5.68)
Adjusted for age, race, BMI
1.57 (0.95–2.59)
1.33 (0.79 –2.25)
2.17 (1.31–3.59)
Adjusted for bioE2c
1.18 (0.68 –2.04)
2.14 (1.30 –3.54)
Adjusted for bioTc
1.50 (0.89 –2.54)
2.23 (1.35–3.69)
Adjusted for SHBGc
1.54 (0.93–2.53)
1.42 (0.84 –2.40)
Full model including bioE2, bioT, and SHBGc
1.43 (0.84 –2.43)
1.26 (0.73–2.20)
2.18 (1.31–3.61)
Full model additionally adjusted for BMDc
1.00 (0.56 –1.77)
1.59 (0.90 –2.81)
2.09 (1.23–3.56)
a HR is for lowest quartile vs. highest three; for bioE2 lowest quartile was less than 11.4 pg/ml (⬍41.8 pmol/liter); for bioT lowest quartile was lessthan 163.5 ng/dl (⬍5.67 nmol/liter); b HR is for highest quartile (SHBG ⱖ59.1 nM) vs. lowest three; c also adjusted for age, race, and BMI; BMDrefers to total hip BMD.
the lowest quartile of bioT was greater among men with
SHBG. For hip fracture risk, the fraction attributed to low
SHBG in the highest quartile (HR 2.10; 95% CI 1.39 –
bioE2 was 0.1%, to low bioT was 2.7%, and to high
3.17; Fig. 4A) than in the lowest three SHBG quartiles (HR
SHBG was 14.6%.
0.99; 95% CI 0.73–1.35; Fig. 4A). These associations re-mained after adjustment for bioE2 and did not appear tobe from a shift in the SHBG distribution; median SHBG
levels did not differ between low and high bioT groups(69.0 vs. 70.4 nM, respectively, P ⫽ 0.7), and adjustment
In this large prospective study of older men, those with the
of the models with an SHBG2 term did not affect the in-
lowest bioE2 or the highest SHBG had higher risks of
teraction. We evaluated whether the stronger association
nonvertebral fracture. BioT had a weak association with
of bioT with fracture risk in the highest SHBG quartile
nonvertebral fracture that disappeared after adjustment
could have been due to particularly low levels of bioT in
for bioE2. The association between bioT and nontrau-
the high SHBG group. The median bioT levels within the
matic fracture risk was stronger and remained after ad-
lowest bioT quartile were slightly lower in the highest
justment for bioE. When high SHBG levels are present,
SHBG quartile compared with the lower quartiles [127.7
low bioT was associated with a substantially increased
vs. 138.7 ng/dl (4.43 vs. 4.81 nmol/liter, respectively,) P ⫽
fracture risk even with bioE2 adjustment. The associations
0.03]. However, in age-, race-, and BMI-adjusted models,
were similar, perhaps slightly stronger, for hip fracture.
even very low levels of bioT were not associated with in-
Total sex steroids were not associated with fracture. These
creases in fracture risk (Fig. 2B). These results indicate that
results have important implications for understanding
low concentrations of bioT impart particular risk in the
how sex steroids and SHBG affect fracture risk and for
presence of high SHBG.
determining the clinical role of these measurements.
Our finding that low bioE2 was independently associ-
Combinatorial effects of estradiol, testosterone,
ated with increased fracture risk extends earlier reports of
and SHBG on fracture risk
estrogen's importance for men's skeletal health (11, 19,
When the combined effects of sex steroid or SHBG lev-
29). Previous studies evaluating the sex steroid-fracture
els were examined, the associations with fracture risk were
association have been inconsistent and limited by cross-
strengthened. The highest nonvertebral fracture risk was
sectional design, low participant and fracture numbers,
in men (n ⫽ 74, 3.7%) in the lowest quartiles of bioT and
and/or RIA-based sex steroid measurements (11, 20, 22,
bioE2 and highest quartile of SHBG (HR 3.39; 95% CI
23, 25, 29, 44). Two recent studies used mass spectrom-
2.19 –5.27; Fig. 4B). Risk estimates were similar or stron-
etry to more accurately measure testosterone and estra-
ger when only nontraumatic fractures were included in the
diol. In the Dubbo cohort, total testosterone had a strong
analyses; in the lowest quartiles of bioT and bioE2 and
and estradiol a weak association with osteoporotic frac-
highest quartile of SHBG, the HR was 4.02 (95% CI 2.54 –
ture risk, (21), but independent effects were not assessed.
6.37). The effects of combining high-risk categories were
Another large, prospective study (MrOS Sweden) (19)
also evident for hip fracture; men with low bioE2 and bioT
found that lower free and total estradiol were associated
and high SHBG levels had a 3.8-fold higher risk of hip
with nonvertebral and vertebral fracture risk. Their results
fracture (95% CI 1.48 –9.92).
are very similar to ours and together provide compellingevidence for estradiol's effects on fracture risk. Attenua-
tion of bioE2's association with fracture by adjustment for
The fraction of nonspine fracture risk attributable to
BMD suggests that estradiol's positive effects on fracture
low bioE2 was 5.7%, 1.5% to low bioT, and 7.7% to high
risk may be due, in part, to an effect on bone density (7, 9,
J Clin Endocrinol Metab, September 2009, 94(9):3337–3346
(log stioa r 1.0
High bioE2 Low bioE2 High bioE2 Low bioE2
High bioT
FIG. 4. Combinations of sex steroids and SHBG and risk of
nonvertebral fracture. A, bioT and SHBG. B, bioT, bioE2, and SHBG.
There were 1079 men in the high bioT, low SHBG category; 397 menin the high bioT, high SHBG category; 392 men in the low bioT, lowSHBG category; and 110 men in the low bioT, high SHBG category.
䡺, Low SHBG; F, high SHBG.
identified similar thresholds of bioE2 below which frac-ture risk was increased [11.4 –12.5 pg/ml (41.8 – 45.9pmol/liter); free estradiol: 0.4 – 0.5 pg/ml (1.47–1.84pmol/liter)]. MrOS Sweden found a similar fracture riskthreshold level [free estradiol: 0.3 pg/ml (1.10 pmol/liter)] (19). Together these results support the hypoth-esis that a threshold range of bioE2 is necessary forskeletal health (45).
High SHBG levels were associated with increased non-
FIG. 3. Spline models for the detection of any nonlinear relationships
vertebral fracture risk, independent of sex steroids and
between sex steroids or SHBG and nonvertebral fracture risk. A, bioE2.
BMD. SHBG has been associated with bone density (22,
B, bioT. C, SHBG. To convert bioavailable estradiol to picomoles perliter, the conversion factor is 3.671; to convert bioavailable
46), bone turnover markers (22, 46), proximal femur ex-
testosterone to nanomoles per liter, the conversion factor is 0.0347.
pansion and bending resistance (47), and fracture risk inmen (19, 22, 46) and women (24). SHBG may directly
11). However, the association remained significant after
influence intracellular signaling via a membrane receptor
BMD adjustment, suggesting additional effects.
that requires SHBG-sex steroid interactions (26, 27) or a
We found a nonlinear association between estradiol
megalin-mediated endocytic pathway that involves un-
and fracture risk. Evaluations using quartile analysis,
bound SHBG (26, 28). Through these pathways, SHBG
spline analysis, and log likelihood cut point analysis
could amplify the effects of sex steroid sufficiency or de-
LeBlanc et al.
Sex Steroids and Fracture in Men
J Clin Endocrinol Metab, September 2009, 94(9):3337–3346
ficiency (26). However, SHBG could also be a marker for
Our results have potential clinical implications. They
nonskeletal factors affecting fracture risk. Lower insulin
affirm the robust and independent effects of bioE2 and
or IGF-I levels could increase SHBG, resulting in the
SHBG in fracture prediction. Moreover, we provide fur-
SHBG-fracture risk association. SHBG increases with age
ther evidence for a threshold level of bioE2, below which
but decreases with obesity. It is affected by frailty and
fracture risk is increased. Hence, estradiol and SHBG mea-
nutritional status. In our study adjustment for age, leg
surements should be valuable in clinical situations. Al-
power, physical activity, BMI, and previous falls did not
though estradiol and SHBG levels are not commonly mea-
alter the association between SHBG and fracture risk.
sured when assessing skeletal health or fracture risk in
Despite strong cellular and animal data suggesting an-
men, our results and those of MrOS Sweden (19) suggest
drogens have positive bone effects, clinical studies offer no
revision of these practices (49). Second, our results sup-
clear evidence of an independent androgen effect on bone
port previous findings that bioavailable or free levels of
mass or fracture (11, 20, 22, 23, 25, 44). Consistent with
sex steroids are more robustly associated with fracture risk
previous reports (19, 21), we found men with low bioT
than are total sex steroid concentrations. Although some
had higher fracture risk, but the association weakened
investigators argue that total T and total E are biologically
when adjusted for bioavailable estradiol. The association
more relevant than bioT or bioE2, our results suggest that
was more robust when only nontraumatic fractures were
bioavailable, not total, levels are associated with fracture
considered, suggesting a stronger link with osteoporotic
risk. It remains common to measure total sex steroid levels
fractures. This could be a reflection of low testosterone's
in clinical situations; however, bioavailable or free levels
effects on fall risk (48), potentially mediated through ex-
may be more appropriate as predictive tools. Given the
traskeletal functions including muscle strength, physical
limitations of the analog free testosterone assays, clinical
activity, and cognition (13–18). Indeed, the association
application of these findings would require more accurate
between low bioT and fracture risk was not attenuated by
and standardized assay methods and development of con-
BMD adjustment, suggesting non-BMD-related factors
sensus concerning assay result use in clinical decision mak-
are important.
ing. Third, the associations we observed were most ap-
We found novel evidence of a bioT-SHBG interaction.
parent when sex steroids and SHBG were considered in
Men with low bioT and high SHBG were at substantially
combination. Men with low bioT and bioE2 and high
higher risk of nonvertebral and hip fracture even after
SHBG levels are at highest risk. If validated, approaches
adjustment for bioE2. Men with low bioT and bioE2 and
that incorporate all three measures into clinical algorithms
high SHBG had even greater risk of nonvertebral (HR 3.4)
should be developed.
and hip fracture (HR 3.8), especially when only nontrau-
This study has several limitations. We did not measure
matic fractures were considered. Thus, bioT, bioE2, and
changes in sex steroids and SHBG over time so cannot
SHBG each play a role in fracture determination, but the
determine how hormonal changes associate with fracture
cumulative effects of sex steroid, and SHBG levels may be
risk. Use of dichotomous cutoffs for sex steroid levels were
most important. Although the findings in MrOS Sweden
based on observed associations with fracture and could
(19) are similar to ours, combinatorial effects of sex ste-
have overestimated the associations. The cohort was rel-
roids and SHBG have rarely been reported. Given these
atively healthy and primarily Caucasian and although
results, combinatorial effects should be evaluated in ad-
similar to more representative populations such as Na-
ditional studies and with other endpoints (e.g. bone loss,
tional Health and Nutrition Examination Survey, caution
body composition changes, cardiovascular events, mor-
should be used in generalizing our results to other groups
tality). However, these results should be interpreted with
of men. The number of hip fractures that occurred during
caution because delineating each hormone's independent
follow-up was relatively small, but nevertheless, the asso-
effect on fracture risk by statistical methods is challenging
ciations between sex steroid and SHBG levels and hip frac-
in the presence of complex interrelationships among
ture risk were robust. Our findings need to be validated in
bioE2, bioT, and SHBG. This is a particular issue in our
other cohorts of older men.
study because bioavailable levels were derived from mass
This study also has considerable strengths. It is one of
action equations that included SHBG. Nevertheless, sev-
the largest to address the association between sex steroids
eral analytical approaches (see Results) provided consis-
and fracture risk in elderly men. Fractures were carefully
tent evidence of a nonartifactual interaction between
ascertained and verified, potentially important confound-
SHBG and bioT. Our findings cannot be considered proof
ing variables were evaluated, and sex steroid measure-
of independent molecular effects of bioavailable sex ste-
ments were performed using gas chromatography/mass
roids and SHBG, but they are consistent with that
spectrometry to avoid inaccuracy at low concentrations
(30, 31). Many participants are over age 80 yr, a segment
J Clin Endocrinol Metab, September 2009, 94(9):3337–3346
of the population that is expanding and is at high fracture
Segre GV, Crowley Jr WF 1989 Increases in bone density during
risk but has not been well studied.
treatment of men with idiopathic hypogonadotropic hypogonad-ism. J Clin Endocrinol Metab 69:776 –783
In summary, men with low bioE2 levels and high SHBG
6. Khosla S 2004 Role of hormonal changes in the pathogenesis of
levels had increased rates of incident fractures. Low bioT
osteoporosis in men. Calcif Tissue Int 75:110 –113
was associated with an increased risk of nontraumatic
7. Khosla S, Melton III LJ, Robb RA, Camp JJ, Atkinson EJ, Oberg AL,
Rouleau PA, Riggs BL 2005 Relationship of volumetric BMD and
fractures and there was an interaction between SHBG and
structural parameters at different skeletal sites to sex steroid levels
bioT; men with low bioT were at higher risk in the pres-
in men. J Bone Miner Res 20:730 –740
ence of high SHBG levels. Men who were in the highest-
8. Khosla S, Melton III LJ, Atkinson EJ, O'Fallon WM 2001 Relation-
risk quartiles for bioT, bioE2, and SHBG had a markedly
ship of serum sex steroid levels to longitudinal changes in bonedensity in young versus elderly men. J Clin Endocrinol Metab 86:
increased fracture risk. Our results suggest that bioavail-
able sex steroid and SHBG measurements may be useful in
9. Szulc P, Munoz F, Claustrat B, Garnero P, Marchand F, Duboeuf F,
the clinical assessment of fracture risk in older men and
Delmas PD 2001 Bioavailable estradiol may be an important deter-
minant of osteoporosis in men: the MINOS study. J Clin Endocrinol
that the physiological implications of hypogonadism
Metab 86:192–199
should be considered in light of possible interactions
10. Slemenda CW, Longcope C, Zhou L, Hui SL, Peacock M, Johnston
among sex steroids and SHBG.
CC 1997 Sex steroids and bone mass in older men. Positive associ-
ations with serum estrogens and negative associations with andro-
gens. J Clin Invest 100:1755–1759
11. Amin S, Zhang Y, Sawin CT, Evans SR, Hannan MT, Kiel DP,
Wilson PW, Felson DT 2000 Association of hypogonadism and
estradiol levels with bone mineral density in elderly men from the
Framingham study. Ann Intern Med 133:951–963
We thank Lori Lambert for her statistical work on previous ver-
12. Beck TJ, Oreskovic TL, Stone KL, Ruff CB, Ensrud K, Nevitt MC,
sions of this manuscript.
Genant HK, Cummings SR 2001 Structural adaptation to changing
skeletal load in the progression toward hip fragility: the study of
Address all correspondence and requests for reprints to: Eric
osteoporotic fractures. J Bone Miner Res 16:1108 –1119
Orwoll, M.D., Bone and Mineral Unit (CR 113), Oregon Health
13. Rudman D, Drinka PJ, Wilson CR, Mattson DE, Scherman F, Cui-
and Science University, 3181 SW Sam Jackson Park Road, Port-
sinier MC, Schultz S 1994 Relations of endogenous anabolic hor-
land Oregon 97239. E-mail: [email protected].
mones and physical activity to bone mineral density and lean body
This work was supported by the Osteoporotic Fractures in
mass in elderly men. Clin Endocrinol (Oxf) 40:653– 661
Men by National Institutes of Health (NIH) funding. The fol-
14. Szulc P, Claustrat B, Marchand F, Delmas PD 2003 Increased risk
of falls and increased bone resorption in elderly men with partial
lowing institutes provided support: the National Institute of Ar-
androgen deficiency: the MINOS study. J Clin Endocrinol Metab
thritis and Musculoskeletal and Skin Diseases, the National In-
stitute on Aging, the National Center for Research Resources,
15. Szulc P, Duboeuf F, Marchand F, Delmas PD 2004 Hormonal and
and NIH Roadmap for Medical Research under the following
lifestyle determinants of appendicular skeletal muscle mass in men:
grant numbers: U01 AR45580, U01 AR45614, U01 AR45632,
the MINOS study. Am J Clin Nutr 80:496 –503
U01 AR45647, U01 AR45654, U01 AR45583, U01 AG18197,
16. Roy TA, Blackman MR, Harman SM, Tobin JD, Schrager M, Metter
U01-AG027810, and UL1 RR024140. Additional support for
EJ 2002 Interrelationships of serum testosterone and free testosterone
these analyses was provided by Merck & Co., Eli Lilly, and
index with FFM and strength in aging men. Am J Physiol Endocrinol
Amgen and NIH Grant AR049828.
Metab 283:E284–E294
Disclosure Statement: E.S.L., C.M.N., L.M.M., J.A.L.,
17. Barrett-Connor E, Goodman-Gruen D, Patay B 1999 Endogenous
K.E.E., A.R.H., G.L., C.O., and E.S.O. have nothing to disclose.
sex hormones and cognitive function in older men. J Clin EndocrinolMetab 84:3681–3685
E.B.-C. has received grant support and/or consulting fees from
18. Moffat SD, Zonderman AB, Metter EJ, Blackman MR, Harman
the National Institutes of Health; Amgen; Eli Lilly and Co.;
SM, Resnick SM 2002 Longitudinal assessment of serum free tes-
Merck & Co., Inc.; Pfizer Pharmaceuticals; Proctor & Gamble;
tosterone concentration predicts memory performance and cogni-
Roche; and Amylin. This financial support does not represent a
tive status in elderly men. J Clin Endocrinol Metab 87:5001–5007
conflict of interest.
19. Mellstrom D, Vandenput L, Mallmin H, Holmberg AH, Lorentzon
M, Oden A, Johansson H, Orwoll ES, Labrie F, Karlsson MK, Ljung-
gren O, Ohlsson C 2008 Older men with low serum estradiol and
high serum SHBG have an increased risk of fractures. J Bone Miner
Res 23:1552–1560
20. Center JR, Nguyen TV, Sambrook PN, Eisman JA 2000 Hormonal
1. Orwoll ES 2003 Men, bone and estrogen: unresolved issues. Osteo-
and biochemical parameters and osteoporotic fractures in elderly
poros Int 14:93–98
men. J Bone Miner Res 15:1405–1411
2. Davidson JM, Chen JJ, Crapo L, Gray GD, Greenleaf WJ, Catania
21. Meier C, Nguyen TV, Handelsman DJ, Schindler C, Kushnir MM,
JA 1983 Hormonal changes and sexual function in aging men. J Clin
Rockwood AL, Meikle AW, Center JR, Eisman JA, Seibel MJ 2008
Endocrinol Metab 57:71–77
Endogenous sex hormones and incident fracture risk in older men:
3. Orwoll E, Lambert LC, Marshall LM, Phipps K, Blank J, Barrett-
the Dubbo Osteoporosis Epidemiology Study. Arch Intern Med 168:
Connor E, Cauley J, Ensrud K, Cummings S 2006 Testosterone and
estradiol among older men. J Clin Endocrinol Metab 91:1336 –1344
22. Legrand E, Hedde C, Gallois Y, Degasne I, Boux de CF, Mathieu E,
4. Cooper C, Campion G, Melton III LJ 1992 Hip fractures in the
Basle MF, Chappard D, Audran M 2001 Osteoporosis in men: a
elderly: a world-wide projection. Osteoporos Int 2:285–289
potential role for the sex hormone binding globulin. Bone 29:90 –95
5. Finkelstein JS, Klibanski A, Neer RM, Doppelt SH, Rosenthal DI,
23. Bjornerem A, Ahmed LA, Joakimsen RM, Berntsen GK, Fonnebo V,
LeBlanc et al.
Sex Steroids and Fracture in Men
J Clin Endocrinol Metab, September 2009, 94(9):3337–3346
Jorgensen L, Oian P, Seeman E, Straume B 2007 A prospective study
study—a large observational study of the determinants of fracture in
of sex steroids, sex hormone-binding globulin, and non-vertebral
older men. Contemp Clin Trials 26:569–585
fractures in women and men: the Tromso Study. Eur J Endocrinol
35. Blank JB, Cawthon PM, Carrion-Petersen ML, Harper L, Johnson
JP, Mitson E, Delay RR 2005 Overview of recruitment for the os-
24. Cummings SR, Browner WS, Bauer D, Stone K, Ensrud K, Jamal S,
teoporotic fractures in men study (MrOS). Contemp Clin Trials
Ettinger B 1998 Endogenous hormones and the risk of hip and
vertebral fractures among older women. Study of Osteoporotic
36. Washburn RA, Smith KW, Jette AM, Janney CA 1993 The Physical
Fractures Research Group. N Engl J Med 339:733–738
Activity Scale for the Elderly (PASE): development and evaluation.
25. Goderie-Plomp HW, van der Klift M, de Ronde W, Hofman A, de
J Clin Epidemiol 46:153–162
Jong FH, Pols HA 2004 Endogenous sex hormones, sex hormone-
37. Sodergard R, Backstrom T, Shanbhag V, Carstensen H 1982 Cal-
binding globulin, and the risk of incident vertebral fractures in el-
culation of free and bound fractions of testosterone and estradiol-
derly men and women: the Rotterdam Study. J Clin Endocrinol
17 to human plasma proteins at body temperature. J Steroid Bio-
Metab 89:3261–3269
chem 16:801– 810
26. Kahn SM, Hryb DJ, Nakhla AM, Romas NA, Rosner W 2002 Sex
38. Heinzl H, Kaider A 1997 Gaining more flexibility in Cox propor-
hormone-binding globulin is synthesized in target cells. J Endocrinol
tional hazards regression models with cubic spline functions. Com-
put Methods Programs Biomed 54:201–208
27. Rosner W, Hryb DJ, Khan MS, Nakhla AM, Romas NA 1999 An-
39. Tableman M, Kim JS 2003 Survival analysis using S: analysis of
drogen and estrogen signaling at the cell membrane via G-proteins
time-to-event data. Boca Raton, FL: CRC Press; 172–175
and cyclic adenosine monophosphate. Steroids 64:100 –106
40. Li R, Chambless L 2007 Test for additive interaction in proportional
hazards models. Ann Epidemiol 17:227–236
28. Hammes A, Andreassen TK, Spoelgen R, Raila J, Hubner N, Schulz
41. Barlow WE, Ichikawa L, Rosner D, Izumi S 1999 Analysis of case-
H, Metzger J, Schweigert FJ, Luppa PB, Nykjaer A, Willnow TE
cohort designs. J Clin Epidemiol 52:1165–1172
2005 Role of endocytosis in cellular uptake of sex steroids. Cell
42. Eide GE, Gefeller O 1995 Sequential and average attributable frac-
tions as aids in the selection of preventive strategies. J Clin Epidemiol
29. Barrett-Connor E, Mueller JE, von Muhlen DG, Laughlin GA,
Schneider DL, Sartoris DJ 2000 Low levels of estradiol are associ-
43. Ruckinger S, von Kries R, Toschke AM 2009 An illustration of and
ated with vertebral fractures in older men, but not women: the Ran-
programs estimating attributable fractions in large scale surveys
cho Bernardo Study. J Clin Endocrinol Metab 85:219 –223
considering multiple risk factors. BMC Med Res Methodol 9:7
30. Taieb J, Mathian B, Millot F, Patricot MC, Mathieu E, Queyrel N,
44. Nyquist F, Gardsell P, Sernbo I, Jeppsson JO, Johnell O 1998 As-
Lacroix I, Somma-Delpero C, Boudou P 2003 Testosterone mea-
sessment of sex hormones and bone mineral density in relation to
sured by 10 immunoassays and by isotope-dilution gas chromatog-
occurrence of fracture in men: a prospective population-based
raphy-mass spectrometry in sera from 116 men, women, and chil-
study. Bone 22:147–151
dren. Clin Chem 49:1381–1395
45. Khosla S 2008 Estrogen and bone: insights from estrogen-resistant,
31. Stanczyk FZ, Cho MM, Endres DB, Morrison JL, Patel S, Paulson
aromatase-deficient, and normal men. Bone 43:414 – 417
RJ 2003 Limitations of direct estradiol and testosterone immuno-
46. Lormeau C, Soudan B, d'Herbomez M, Pigny P, Duquesnoy B, Cor-
assay kits. Steroids 68:1173–1178
tet B 2004 Sex hormone-binding globulin, estradiol, and bone turn-
32. Siekmann L 1979 Determination of steroid hormones by the use of
over markers in male osteoporosis. Bone 34:933–939
isotope dilution-mass spectrometry: a definitive method in clinical
47. Kaptoge S, Dalzell N, Folkerd E, Doody D, Khaw KT, Beck TJ, Lov-
chemistry. J Steroid Biochem 11:117–123
eridge N, Mawer EB, Berry JL, Shearer MJ, Dowsett M, Reeve J 2007
33. Lawson AM, Gaskell SJ, Hjelm M 1985 International Federation of
Sex hormone status may modulate rate of expansion of proximal femur
Clinical Chemistry (IFCC), Office for Reference Methods and Ma-
diameter in older women alongside other skeletal regulators. J Clin
terials (ORMM). Methodological aspects on quantitative mass
Endocrinol Metab 92:304–313
spectrometry used for accuracy control in clinical chemistry. J Clin
48. Orwoll E, Lambert LC, Marshall LM, Blank J, Barrett-Connor E,
Chem Clin Biochem 23:433– 441
Cauley J, Ensrud K, Cummings SR 2006 Endogenous testosterone
34. Orwoll E, Blank JB, Barrett-Connor E, Cauley J, Cummings S,
levels, physical performance, and fall risk in older men. Arch Intern
Ensrud K, Lewis C, Cawthon PM, Marcus R, Marshall LM, McGowan
Med 166:2124 –2131
J, Phipps K, Sherman S, Stefanick ML, Stone K 2005 Design and base-
49. Gennari L, Khosla S, Bilezikian JP 2008 Estrogen and fracture risk
line characteristics of the osteoporotic fractures in men (MrOS)
in men. J Bone Miner Res 23:1548 –1551
Source: http://www.nutrologia.med.br/skin/upload/2014011717062385.pdf
alturos.co.uk
© Alturos Ltd 2006-2013 Applying Process Improvement in an NHS Pharmacy to Reduce Cost, Increase Productivity and Create a Better Working Environment In July 2010, the Pharmacy at one site of one of London's largest NHS Foundation Trust's, started applying Lean working methods. Since that time, a number of projects have developed. This article
americanrtl.org
the abortion pill by W. David Hager, M.D. A positive pregnancy test is one of the most life-changing moments for a woman. Never is it more important to base your decisions on accurate information. Try to think beyond the pressures you face right now and consider the long-term impact of your choices. You may have considered —or someone around you may have suggested—having an abortion.