New.virbac.cz
Vet Res Commun (2011) 35:501–509DOI 10.1007/s11259-011-9493-7 Analysis of epidermal lipids in normal and atopic dogs,before and after administration of an oral omega-6/omega-3 fatty acid feed supplement. A pilot study Iuliana Popa & Didier Pin & Nathalie Remoué &Bilal Osta & Sylvie Callejon & Emilie Videmont &Hugues Gatto & Jacques Portoukalian & Marek Haftek Accepted: 12 July 2011 / Published online: 23 July 2011 # Springer Science+Business Media B.V. 2011 Abstract Alterations of the lipid expression in the skin of human and canine atopicsubjects may be one of the key factors in the disease development. We have analyzed theultrastructure of the clinically uninvolved skin of atopic dogs and compared it with the lipidcomposition of their tape-stripped stratum corneum (SC). The effect of a 2 month treatmentof atopic dogs by food supplementation with a mixture of essential fatty acids wasevaluated on skin samples taken before and after the treatment period. Electron microscopyrevealed that the non-lesional skin of atopic dogs exhibited an abnormal and largelyincomplete structure of the lamellar lipids with little cohesion between the corneocytestrata. The SC of atopic dogs was characterized by a significant decrease in the lipid contentwhen compared to the healthy controls. Following oral supplementation with the mixture ofessential fatty acids, the overall lipid content of the SC markedly increased. This featurewas observed both with the free and, most importantly, with the protein-bound lipids(cholesterol, fatty acids and ceramides), the latter constituting the corneocyte-boundscaffold for ordinate organisation of the extracellular lipid bi-layers. Indeed, the semi-quantitative electron microscopy study revealed that the treatment resulted in a significantlyimproved organization of the lamellar lipids in the lower SC, comparable to that of the I. Popa N. Remoué B. Osta S. Callejon J. Portoukalian M. HaftekLaboratory for Dermatological Research EA4169 "Normal and Pathological Functions of the SkinBarrier", Université Lyon 1, Lyon, France D. Pin E. VidemontDermatology–Cancerology Unit, VetAgro Sup Lyon Campus, Marcy L'Etoile, France H. GattoVirbac SA, Carros, France M. Haftek (*)Laboratoire de Recherche Dermatologique, EA4169, UCBL1, 8, Av. Rockefeller, 69373 Lyon, Francee-mail: [email protected] Present Address:I. PopaUMR CNRS 8612, University of Paris XI, 92290 Chätenay-Malabry, France Vet Res Commun (2011) 35:501–509 healthy dogs. Our results indicate the potential interest of long-term alimentarysupplementation with omega-6 and omega-3 essential fatty acids in canine atopicdermatitis.Keywords Canine atopic dermatitis . Lipid analysis . Stratum corneum . Feed supplement .
Dogs Atopic dermatitis Essential fatty acids According to the latest criteria, canine atopic dermatitis (AD) is defined as a geneticallydetermined, allergic, inflammatory, and pruritic skin disorder, with characteristic clinicalfeatures, most commonly associated with IgE antibodies to environmental allergens(Willemse Griffin and DeBoer It is a common condition estimated to affect10–15% of the canine population. Clinical and immunological studies of canine ADsuggest that canine AD is probably one of the closest animal models to human AD (Hillierand Olivry ). In humans, epidermal barrier dysfunction provoked by loss-of-functionmutations in the filaggrin gene has been incriminated in the predisposition to develop AD(Sandilands et al. Jakasa et al. This was the first documented proof ofimplication of the altered permeability barrier in the pathogenesis of AD (Elias et al. However, abnormal lipid expression in the stratum corneum (SC) of uninvolved skin of ADpatients has also been reported (Imokawa et al. ; Di Nardo et al. Macheleidt etal. ) and is likely to be another factor predisposing to the disease because itsoccurrence seems to be dissociated from the presence of filaggrin mutations (Jungersted etal. ). Indeed, a subgroup of canine AD can be defined that do not depend on thefilaggrin gene mutations (O'Regan et al. There is no clear-cut evidence published so far that canine AD is associated with defective filaggrin expression (Chervet et al. On the contrary, linkage studies in WestHighland White Terriers indicate the absence of such correlation (Barros Roque et al. However, SC lipid abnormalities have been reported. Indeed, both continuity and overallthickness of the SC intercellular lipid lamellae, which are prerequisite for the proper barrierfunction, were significantly decreased in the non-lesional skin of dogs with AD (Inman etal. Piekutowska et al. ). These morphological findings have been supported bybiochemical analyses showing abnormal composition of the SC lipids, and morespecifically ceramides, in AD dogs (Reiter et al. Shimada et al. ; Popa et al.
Popa et al., (in preparation)) and by functional assays testifying to an increasedpermeability of the skin barrier in this disease (Shimada et al. ). Once defined as a possiblecausative factor in AD, SC lipids became the target of therapies aimed at improving theepidermal barrier function. Topical application of a mixture of lipids necessary for re-structuring of the SC lipid lamellae has been successfully used in dogs and resulted in bothstructural and clinical improvement (Piekutowska et al. ; Popa et al. , ). A feedsupplement containing essential fatty acids (EFA) and vitamin E has been designed to helprestore the cutaneous integrity by normalizing the lipid metabolism and thus improving thequality of the lipids present in the skin. Its potential influence on the composition andstructure of the SC intercellular lipids in AD dogs has been addressed in the present study.
Vet Res Commun (2011) 35:501–509 Materials and methods The study was conducted in compliance with the Procedures of Good Clinical Practice, asdetailed in the European Commission Note.
Client-owned dogs with AD were eligible for inclusion in the study. The diagnostic of AD was made on the basis of a compatible history and fulfillment of the currently acceptedclinical criteria proposed by Willemse () and redefined by Favrot et al. (). Allsimilar-appearing skin diseases with pruritus, such as sarcoptic mange, flea allergydermatitis, bacterial pyoderma and Malassezia dermatitis were carefully ruled out,according to standard diagnostic and treatment methods. Dogs with an uncontrolled skinor auricular bacterial or fungal infection or with uncontrolled flea allergy dermatitis wereexcluded from the study. Before the beginning of the study, several local and systemictreatments have been interrupted. Parenteral administration of long-lasting corticosteroids,oral and topical administration of corticosteroids were discontinued for at least 8, 3 and2 weeks, respectively; EFA administration was discontinued for at least 8 weeks;administration of antihistamines, cyclosporine or any other immunosuppressive drug wasdiscontinued for at least 2, and 8 weeks, respectively.
Five dogs from different breeds clinically diagnosed with AD were selected for this study (Table Five healthy adult female Beagle dogs (5–6 years of age) were used as thereference normal control.
Before the beginning of the study, several local and systemic treatments have been interrupted.
Parenteral administration of long-lasting corticosteroids, oral and topical administration ofcorticosteroids were discontinued for at least 8, 3 and 2 weeks, respectively; EFA administrationwas discontinued for at least 8 weeks; administration of antihistamines, cyclosporine or any otherimmunosuppressive drug was discontinued for at least 2, and 8 weeks, respectively.
One chlorhexidine-containing shampoo of the skin lesional areas per week and the use of an ear cleanser without corticosteroid once or twice a week were authorized. In all dogs, a topical fleaadulticide with minimum 1-month duration of activity was applied monthly throughout the study.
Owners of the dogs considered for inclusion in the study received detailed information on the study and provided a written informed consent.
The protocol was approved by the Animal Care and Use Committee of the Veterinary School of Lyon.
The AD dogs were administered orally one dose of 4 ml per day for dogs of less than10 kg or one dose of 8 ml per day for dogs with body weight over 10 kg ofMegaderm®/EFA-Z® (Virbac S.A.; Carros, France), every day for 8 weeks. This feed Table 1 Breed, age, and sex of the AD dogs studied West Highland White Terrier Vet Res Commun (2011) 35:501–509 supplement is composed of the EFA, omega-6 (linoleic acid 350 mg/ml, gamma-linolenic acid 45 mg/ml) and omega-3 (eicosapentaenoic acid 25 mg/ml, docosahex-aenoic acid 28 mg/ml), mixed 5:1 (v/v), and contains vitamin E 3.8 UI/mL. The 8 weeksof treatment were followed by 1 week of wash-out.
To chemically and structurally analyse the epidermal lipids contained in the stratumcorneum, before and after the administration of EFA and thus evaluate the effect of foodsupplementation, the stratum corneum was harvested by sequential tape-stripping and skinbiopsies were performed in the dogs before the study and after 1 week of wash-out. As thestudy was aimed at the evaluation of clinically non-involved skin, we have chosen thelateral thorax region, which is less frequently involved during canine AD, for comparisonsbetween AD and control groups.
Chemical analysis of the SC lipids Consecutive adhesive tape-strips (Scotch® Magic™ tape; 3M, Cergy-Pontoise, France)were applied on the same area of non-lesional skin at the lateral aspect of the thorax. Beforetape stripping, hairs in the area were carefully clipped with a shaving machine to the lengthof <2 mm. The tape was pressed to the skin with fingertips for 5 s. before rapid removal.
Approximately 12 consecutive strips per area were needed to reach the viable epidermischaracterized by a glistening skin surface.
SC lipids were extracted from tape strips using previously described methods (Popa et al. , ). Briefly, hexane/isopropyl alcohol mixture (4:1, v/v) was used to releasethe corneocytes from tape strips. Free intercellular lipids were extracted using chloroform/methanol (2:1 v/v mixture, two times) and then methanol alone (Extraction I). The cellpellet resulting from this first extraction was subjected to mild saponification with 0.1NKOH in methanol/water (10:1 v/v) for 2 h at 50°C (Wertz et al. After neutralizationwith 1N HCl, the protein residue was recovered by centrifugation and assayed by theCoomassie blue/Bradford method, whereas the lipids released from the cornified envelopeswere harvested from the supernatants using phase separation after addition of distilled waterand chloroform in appropriate proportions (Extraction II). Free (FL) and protein-boundlipids (PB) from each extraction were recovered by solvent evaporation, taken up in 0.5 mLof diethylether and separated into different classes on a LC-NH2 silica gel column(Supelco; L'Isle d'Abeau, France). All fractions were evaporated to dryness under nitrogenatmosphere and analysed with high performance thin layer chromatography. To this end, thelipid samples applied on silica gel plates were migrated in the following solvent systemssuitable for each lipid class: hexane–diethylether–acetic acid 70:30:1 (by volume) forneutral lipids and fatty acids; chloroform–methanol 50:3 (v/v) for ceramides. The lipidswere visualized by charring for 5 min at 150°C after spraying with a reagent made of 3%cupric acetate in 8% phosphoric acid. Fractions containing ceramides were pooled andhydrolysed to release the long-chain bases, thus allowing quantification of shingolipids bythe fluorescamine method (Naoi et al. ). Fractions containing cholesterol and fattyacids were also pooled. The distribution of components was determined by scanningdensitometry with a CS-930 Chromatoscan (Shimadzu; Kyoto, Japan). For definition ofceramide subclasses we have referred to the nomenclature defined by Motta et al. ( Quantitative results obtained on normal and atopic canine SC were compared using Mann–Whitney test, P≤0.05 being accepted as significant.
Vet Res Commun (2011) 35:501–509 Structural analysis of the SC lipids Punch biopsies (6 mm) were taken, under local anaesthesia, from a non-lesional zone of thelateral aspect of the thorax, next to the area of tape-stripping. Skin fragments were cut intosmall blocks and fixed in 1% glutaraldehyde/4% paraformaldehyde in phosphate bufferedsaline (PBS, pH 7.4) for 24 h. After washings in PBS, some blocks were post-fixed in 1%osmium tetroxide, the others in 0.5% ruthenium tetroxide for 2 h. All blocks were furtherwashed in PBS, dehydrated in graded ethanol series, impregnated with and embedded inEpon resins according to standard procedures. Ultrathin sections counterstained with uranylacetate and lead citrate were observed in a transmission electron microscope at 60 kV.
Digital micrographs of the lowermost stratum corneum compactum were taken at 60,000xand 125,000x and analyzed using camera-dedicated software (AnalySIS, Olympus SoftImaging Solutions; Hamburg, Germany). Specifically, we measured the section surface ofthe SC extracellular spaces and, within these areas, defined the surface occupied byextracellular lipids organized in bi-layers. Several optical fields on every specimen werethus analyzed and the results were expressed as mean percentage of the extracellular spaceoccupied by the structured lipid lamellae. Statistical comparison between the differentgroups of biopsies was performed using Chi2 test.
Quantitative analysis of the high performance thin layer chromatography of SC lipidsextracted from the non-involved skin of atopic dogs has demonstrated a remarkabledecrease in all three protein-bound lipid classes studied, i.e.: ceramides, cholesterol and freefatty acids, when compared to the healthy controls (Fig. Within the free intercellularlipids, a significant decrease was only noted in the ceramide fractions. After 2 months ofMegaderm®/EFA-Z® supplementation, the lipid contents have changed significantly (Figs.and ). It returned within the control levels for protein-bound cholesterol and fatty acidsand, remarkably, for free intercellular ceramides. However, the protein-bound fraction ofceramides, although significantly increased when compared to the situation from before thetreatment, remained well below the values found in healthy dogs. Additionally, 2-monthsupplementation with EFA resulted in a considerable increase in the SC free cholesterol andfree fatty acids, exceeding the levels found in both normal dogs and in non-involved atopicskin.
Quantitative analysis of the SC lipids structured in bi-layers was performed using transmission electron microscopy of the ruthenium tetroxide-stained skin biopsies (Fig. In non-involved skin of the atopic dogs, only a small fraction of the first two extracellularspaces of the SC was filled with the lipid lamellae. This was in evident contrast with thesituation observed in normal skin of the control dogs, where the lipid lamellae occupied90% of the area. The treatment of AD dogs with EFA resulted in a highly significantincrease in this respect, from 26% to 59%, but the situation after the treatment remained stilldeficient when compared to the normal (P<0.05).
Roughly equimolar proportions of ceramides, cholesterol, and free fatty acids are necessaryfor building the efficient lipid-dependant permeability barrier in the SC (Elias ;

Vet Res Commun (2011) 35:501–509
Fig. 1 Densitometry of the SC lipids extracted from non-involved skin of atopic dogs before and aftertreatment with the EFA supplement. Free and protein-bound lipids were extracted from the SC tape-strips, separated, visualized, and quantified as described in the The samples ofceramides (A), cholesterol (B), and fatty acids (C) from before (BF) and after (AF) treatment arecompared. Atopic dogs show a remarkable decrease in free and protein-bound ceramides before thetreatment, compared to normal controls (N). After 2 months of supplementation, free ceramides return tonormal but not the cell envelope-bound species. Initially depressed, protein-linked cholesterol and fattyacids return to the normal values after the treatment. EFA supplementation induces also a considerableincrease in the free cholesterol and fatty acid fractions. The lipid quantities are expressed in μg per mg ofdry weight SC protein used for extraction; mean ± SD; n=5; Mann–Whitney test.
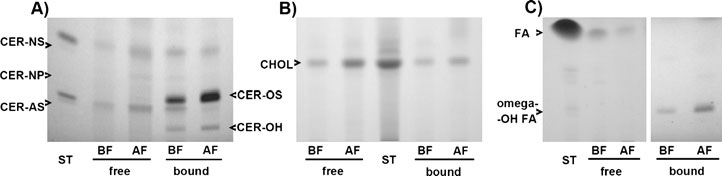
Fig. 2 Representative results of high performance thin layer chromatography of the SC lipids from the non-involved skin of a dog with AD, showing remarkable modifications in the lipid composition after EFAsupplementation. Free and protein-bound lipids were extracted from the SC strips, separated and visualized,as described in the . The samples of ceramides (A), cholesterol (B), and fatty acids (C)from before (BF) and after (AF) treatment are compared. There is a visible increase after the treatment inamounts of the extracted free and protein-bound ceramides, as well as free cholesterol and free fatty acids.
The composition of visualized ceramide (CER) molecules is as follows: NS = normal fatty acid linked tosphingosine; NP = normal fatty acid linked to phytosphingosine; AS = alpha-hydroxy fatty acid linked tosphingosine; OS = omega-hydroxy fatty acid linked to sphingosine; OH = omega-hydroxy fatty acid linkedto 6-hydroxy sphingosine

Vet Res Commun (2011) 35:501–509
Fig. 3 Quantitative evaluation of the lipids structured in lamellar sheets occupying the first two intercellularspaces of the dogs' SC. Although the treatment with EFA results in a significant increase in the lipid lamellaein non-involved atopic skin (BF before; AF after), the values reached remain significantly lower than thosefound in the reference healthy animals (N). Each bar represents the mean value, error bars indicate ± SD; n=5; Chi2 test
Bouwstra et al. ). Changes in this delicate balance observed in skin pathology, andwhich can be reproduced experimentally, lead to the "leaky" SC, with all the functional andclinical consequences (Bouwstra et al. Holleran and Takagi In addition, inorder to build the barrier, the extracellular lipids have to be correctly structured in lipid bi-layers (Garson et al. ; Potts and Francoeur ; Pilgram et al. Lipid envelopecomposed of a monolayer of omega-hydroxylated ceramide and fatty acid moleculescovalently cross-linked to the surface of corneocyte's protein envelope serves as a templatefor organization of the extracellular lipids and thus is essential for ordinate construction ofthe SC lipid bi-layers and barrier function (Wertz et al. Behne et al. ; Meguro etal. ). Indeed, not only the overall decrease of the SC ceramides (Imokawa et al. ;Di Nardo et al. ) but also a significant reduction in omega-hydroxylated ceramides hasbeen reported in human AD (Macheleidt et al. ). The compositional and structural lipiddeficiency in the non-lesional SC of atopic dogs, previously reported and also observed inthe present study emerges, therefore, as a plausible constitutive factor leading to theincreased skin barrier permeability and, in turn, to the development and maintenance of ADlesions (Inman et al. Olivry and Hill Piekutowska et al. Reiter et al.
Shimada et al. ; Popa et al. ,
The fact that both the overall lipid quantities and their ultrastructural arrangement in
multiple molecular layers increase significantly after supplementation with EFA isnoteworthy (Menon Improvement in the expression of protein-bound ceramides isthe most remarkable feature, as it may be responsible for the observed accumulation of thelamellar lipids (Ponec et al. However, the level of organisation of the lipid layers inthe most profound part of the SC after 2 months of EFA supplementation remains far fromnormal. This may be due to the imperfect proportions between the SC lipids induced by thetreatment. Indeed, free fatty acids and cholesterol increase approximately two times morethan ceramides in the lipid fraction not bound to proteins, and this is also the case ofprotein-bound fatty acids. Assessment of the lipid profile in the SC using in parallel highperformance thin layer chromatography and transmission electron microscopy can providevaluable data indicating the SC barrier competence in healthy and diseased skin.
Reasons underlying lipid defects in the skin of AD dogs remain unknown but may be
related to some constitutive metabolic defect, as they occur preferentially in certain breeds.
Sustained EFA supplementation exerts most probably influence on keratinocyte metabo-
Vet Res Commun (2011) 35:501–509
lism, fostering production of the barrier lipids (Mao-Qiang et al. Such a mechanismhas already been suggested to explain the beneficial effects of topically appliedsphingolipids in canine AD (Piekutowska et al.
The clinical evolution observed in AD dogs treated with EFA can be at least partially
ascribed to the improvement in the composition and structure of the SC lipids (Proksch etal. ). The structural and quantitative changes in the SC barrier lipids induced by thisfeed supplementation in the non-lesional skin suggest an improvement of the barrierfunction of the epidermis. If this is associated with a better resistance to transcutaneouspenetration of foreign agents susceptible of driving atopic inflammatory responses remainsto be studied. Based on the obtained results, it would be extremely interesting to perform anadditional study in which control dogs, breed and age-paired with the AD group, wouldreceive the Megaderm supplementation. Also, additional evaluation of potential alternatetargets of the feed supplementation, other than epidermal ceramides, e.g., eicosanoidsynthesis pathway and cytokine production, could be helpful for a better comprehension ofthe mechanisms involved in the clinically observed improvement.
Acknowledgements This study was supported by the European COST action BM0903 (SkinBAD, skinbarrier in atopic dermatitis) and the European Epidermal Barrier Research Network (E²BRN).
Electron microscopy samples were examined at the Centre Technologique des Microstructures (CTμ) de
l'Université de Lyon1, La Doua, Villeurbanne, France.
The results reported in this paper have been partially presented at the Sixth World Congress of Veterinary
Dermatology, Hong Kong, November 2008 (abstract in Vet. Dermatol. 2008; 19 (suppl 1): 68) and at theEuropean Society for Dermatological Research meeting, Helsinki, September 2010 (abstract in J InvestDermatol 130: S13, 2010).
Conflict of interest This study was partially financed by Virbac S.A., France.
Barros Roque J, O'Leary CA, Kyaw-Tanner M et al (2009) Haplotype sharing excludes canine orthologous
filaggrin locus in atopy in West Highland White Terriers. Anim Gen 40:788–794
Behne M, Uchida Y, Seki T et al (2000) Omega-hydroxyceramides are required for corneocyte lipid
envelope (CLE) formation and normal epidermal permeability barrier function. J Invest Dermatol114:185–192
Bouwstra JA, Pilgram GSK, Ponec M (2006) Structure of the skin barrier. In: Elias PM, Feingold KR (eds)
Skin barrier. Taylor & Francis, New York, pp 65–96
Chervet L, Galichet A, McLean WHI, Chen H, Suter MM, Roosje PJ, Müller EJ (2010) Missing C-terminal
filaggrin expression, NFkappaB activation and hyperproliferation identify the dog as a putative model tostudy epidermal dysfunction in atopic dermatitis. Exp Dermatol 19:e343–e346
Di Nardo A, Wertz P, Giannetti A et al (1998) Ceramide and cholesterol composition of the skin of patients
with atopic dermatitis. Acta Derm Venereol 78:27–30
Elias PM (2006) Improving barrier function. In: Elias PM, Feingold KR (eds) Skin barrier. Taylor & Francis,
New York, pp 591–600
Elias PM, Hatano Y, Williams M (2008) Basis for the barrier abnormality in atopic dermatitis: outside-inside-
outside pathogenic mechanisms. J Allergy Clin Immunol 121:1337–1343
Favrot C, Steffan C, Seewald W et al (2010) A prospective study on the clinical features of chronic canine
atopic dermatitis and its diagnosis. Vet Dermatol 21:23–31
Garson JC, Doucet J, Levèque JL et al (1991) Oriented structure in human stratum corneum revealed by X-
ray diffraction. J Invest Dermatol 96:43–49
Griffin CE, DeBoer DJ (2001) The ACVD task force on canine atopic dermatitis (XIV): clinical
manifestations of canine atopic dermatitis. Vet Immunol Immunopathol 81:255–269
Vet Res Commun (2011) 35:501–509
Hillier A, Olivry T (2004) Spontaneous canine model of atopic dermatitis. In: Chan LS (ed) Animal models
of human inflammatory skin diseases. CRC, Boca Raton, pp 353–370
Holleran WM, Takagi Y (2006) Stratum corneum lipid processing: the final steps in barrier formation. In:
Elias PM, Feingold KR (eds) Skin barrier. Taylor & Francis, New York, pp 231–260
Imokawa G, Abe A, Jin K et al (1991) Decreased level of ceramides in stratum corneum of atopic dermatitis:
an etiologic factor in atopic dry skin? J Invest Dermatol 96:523–526
Inman AO, Olivry T, Dunston SM et al (2001) Electron microscopic observations of stratum corneum
intercellular lipids in normal and atopic dogs. Vet Pathol 38:720–723
Jakasa I, Koster ES, Calkoen F et al (2011) Skin barrier function in healthy subjects and patients with atopic
dermatitis in relation to filaggrin loss-of-function mutations. J Invest Dermatol 131:540–542
Jungersted JM, Scheer H, Mempel M et al (2010) Stratum corneum lipids, skin barrier function and filaggrin
mutations in patients with atopic eczema. Allergy 65:911–918
Macheleidt O, Kaiser HW, Sandhoff K (2002) Deficiency of epidermal protein-bound omega-
hydroxyceramides in atopic dermatitis. J Invest Dermatol 119:166–173
Mao-Qiang M, Brown BE, Wu-Pong S et al (1995) Exogenous nonphysiologic vs physiologic lipids.
Divergent mechanisms for correction of permeability barrier dysfunction. Arch Dermatol 131:809–816
Meguro S, Arai Y, Masukawa Y et al (2000) Relationship between covalently bound ceramides and
transepidermal water loss (TEWL). Arch Dermatol Res 292:463–468
Menon GK (2006) What makes a good barrier? Adaptive features of vertebrate integument. In: Elias PM,
Feingold KR (eds) Skin barrier. Taylor & Francis, New York, pp 211–222
Motta S, Monti M, Sesana S et al (1993) Ceramide composition of the psoriatic scale. Biochem Biophys
Acta 1182:147–151
Naoi M, Lee YC, Roseman S (1974) Rapid and sensitive determination of sphingosine bases and
sphingolipids with fluorescamine. Anal Biochem 58:571–577
Olivry T, Hill PB (2001) The ACVD task force on canine atopic dermatitis: is the epidermal lipid barrier
defective? Vet Immunol Immunopathol 81:215–218
O'Regan GM, Kemperman PM, Sandilands A et al (2010) Raman profiles of the stratum corneum define 3
filaggrin genotype-determined atopic dermatitis endophenotypes. J Allergy Clin Immunol 126:574–580
Piekutowska A, Pin D, Rème CA et al (2008) Effects of a topically applied preparation of epidermal lipids on
the stratum corneum barrier of atopic dogs. J Comp Pathol 138:197–203
Pilgram GSK, Vissers DCJ, van der Meulen H, Pavel S, Lavrijsen SPM, Bouwstra JA, Koerten HK (2001)
Aberrant lipid organization in stratum corneum of patients with atopic dermatitis and lamellar ichthyosis.
J Invest Dermatol 117:710–717
Ponec M, Boelsma E, Weerheim A (2000) Covalently bound lipids in reconstructed human epithelia. Acta
Derm Venereol (Stockh) 80:89–93
Popa I, Thuy LH, Colsch B et al (2010) Analysis of free and protein-bound ceramides by tape stripping of
stratum corneum of dogs. Arch Dermatol Res 302:639–644
Popa I, Remoué N, Thuy LH et al (2011) Atopic dermatitis in dogs is associated with a high heterogeneity in
the distribution of protein-bound lipids within the stratum corneum. Arch Dermatol Res. doi:
Potts RO, Francoeur ML (1991) The influence of stratum corneum morphology on water permeability. J
Invest Dermatol 96:495–499
Proksch E, Jensen JM, Elias PM (2003) Skin lipids and epidermal differentiation in atopic dermatitis. Clin
Dermatol 21:134–144
Reiter LV, Torres SMF, Wertz PW (2009) Characterization and quantification of ceramides in the nonlesional
skin of canine patients with atopic dermatitis compared with controls. Vet Dermatol 20:260–266
Sandilands A, Terron-Kwiatkowski A, Hull PR et al (2007) Comprehensive analysis of the gene encoding
filaggrin uncovers prevalent and rare mutations in ichthyosis vulgaris and atopic eczema. Nat Genet39:650–654
Shimada K, Yoon JS, Yoshihara T et al (2009) Increased transepidermal water loss and decreased ceramide
content in lesional and non-lesional skin of dogs with atopic dermatitis. Vet Dermatol 20:541–546
Wertz PW, Madison KC, Downing DT (1989) Covalently bound lipids of human stratum corneum. J Invest
Dermatol 92:109–111
Willemse T (1986) Atopic skin disease: a review and a reconsideration of diagnostic criteria. J Small Anim
Pract 27:771–778
Source: http://new.virbac.cz/files/mala-zvirata/article.pdf
Issn 2320-5407 international journal of advanced research (2014), volume 2, issue 11, 660-664
ISSN 2320-5407 International Journal of Advanced Research (2014), Volume 2, Issue 11, 660-664 Journal homepage: INTERNATIONAL JOURNAL OF ADVANCED RESEARCH RESEARCH ARTICLE Spironoctone in Psoriatic Arthritis; Safety, Efficacy and Effect on Disease Activity Inderjeet Verma,1 Ashit Syngle,2 Pawan Krishan1
Vehicles
REQUEST FOR PROPOSAL BID SHEET(S) RFP 0601-2013: GROUP HEALTH INSURANCE EAST TEXAS COUNCIL OF GOVERNMENTS SPECIFICATIONS EAST TEXAS COUNCIL OF GOVERNMENTS HUMAN RESOURCES DEPARTMENT OPENING DATE: TUESDAY, MARCH 19, 2013 10:00 AM CST EAST TEXAS COUNCIL OF GOVERNMENTS • HUMAN RESOURCES DEPARTMENT • 3800 STONE ROAD • KILGORE, TEXAS 75662