Microsoft word - doa multi-panels rev17 en_no.doc

DIALAB Produktion und Vertrieb von chemisch – technischen Produkten und Laborinstrumenten Gesellschaft m.b.H.
A – 2351 Wiener Neudorf, Austria, IZ-NÖ Süd, Hondastrasse, Objekt M55
Phone: ++43 (0) 2236 660910-0, Fax: ++43 (0) 2236 660910-30, e-mail: [email protected]
"DIAQUICK" Multi-Drug Panels
This test will detect other related compounds, please refer to the Analytical Specificity table in this insert. This assay provides only a preliminary analytical test result. A more specific
for human urine samples
alternate chemical method must be used in order to obtain a confirmed analytical result. Gas chromatography/mass spectrometry (GC/MS) is the preferred confirmatory method. Clinical
Multi-3 Drug Panel BZO,COC,MOP
consideration and professional judgment should be applied to any drug of abuse test result,
Cont.: 30 panels, individually packed (30x REF Z06576B)
particularly when preliminary positive results are obtained. For in vitro diagnostic use only
Cont.: 1 panel, individually packed
TEST PRINCIPLE
Multi-3/1 Drug Panel BUP, MOP, MTD
Cont.: 30 panels, individually packed (30x REF Z09577B)
The "DIAQUICK" Multi-Drug Panels (urine) are immunoassays based on the principle of
Cont.: 1 panel, individually packed
competitive binding. Drugs which may be present in the urine specimen compete against their respective drug conjugate for binding sites on their specific antibody. During testing, a
Multi-4 Drug Panel AMP,COC,MOP,THC
Cont.: 30 panels, individually packed (30x REF Z02575B)
urine specimen migrates upward by capillary action. A drug, if present in the urine specimen
Cont.: 1 panel, individually packed
below its cut-off concentration, will not saturate the binding sites of its specific antibody coated on the particles. The antibody coated particles will then be captured by the immobi-
Multi-5 Drug Panel BZO,COC,MET,MOP,THC
lized drug conjugate and a visible colored line will show up in the test line region of the
Cont.: 30 panels, individually packed (30x REF Z05236B)
specific drug strip. The colored line will not form in the test line region if the drug level is
Cont.: 1 panel, individually packed
above its cut-off concentration because it will saturate all the binding sites of the antibody
Multi-5/3 Drug Panel AMP,COC,MET,MOP,THC
coated on the particles. A drug-positive urine specimen will not generate a colored line in the
Cont.: 30 panels, individually packed (30x REF Z06502B)
specific test line region of the strip because of drug competition, while a drug-negative urine
Cont.: 1 panel, individually packed
specimen or a specimen containing a drug concentration less than the cut-off will generate a
Multi-5/4 Drug Panel AMP,COC,MDMA,MOP,THC
line in the test line region. To serve as a procedural control, a colored line will always appear
Cont.: 30 panels, individually packed (30x REF Z11504B)
at the control line region indicating that proper volume of specimen has been added and
Cont.: 1 panel, individually packed
membrane wicking has occurred.
Multi-5/6 Drug Panel AMP,BZO,COC,MOP,THC
WARNINGS AND PRECAUTIONS
Cont.: 30 panels, individually packed (30x REF Z06506B)
For medical and other in vitro diagnostic use only. Do not use after the expiration date.
Cont.: 1 panel, individually packed
The test panel should remain in the sealed pouch until use.
Multi-6 Drug Panel BZO,COC,MET,MOP,MTD,THC
All specimens should be considered potential y hazardous and handled in the same
Cont.: 30 panels, individually packed (30x REF Z98907B)
manner as an infectious agent.
Cont.: 1 panel, individually packed
The used test panels should be discarded according to federal, state and local regula-
Multi-6/1 Drug Panel AMP,BZO,COC,MET,MOP,THC
Cont.: 30 panels, individually packed (30x REF Z03220B)
REAGENTS
Cont.: 1 panel, individually packed
Each test line contains anti-drug mouse monoclonal antibody and corresponding drug-
Multi-6/3 Drug Panel BUP, BZO, COC, MTD, OPI, THC
protein conjugates. The control line contains goat anti-rabbit IgG polyclonal antibodies and
Cont.: 30 panels, individually packed (30x REF Z08930B)
Cont.: 1 panel, individually packed
Multi-6/4 Drug Panel AMP,BUP,BZO,MET,MOP,THC
Cont.: 30 panels, individually packed (30x REF Z08940B)
The "DIAQUICK" Multi-Drug Panels can be stored refrigerated or at room temperature (2-
Cont.: 1 panel, individually packed
30°C). The test panel is stable through the expiration date printed on the sealed pouch. The test panel must remain in the sealed pouch until use. DO NOT FREEZE. Do not use beyond
Multi-6/6 Drug Panel BUP,COC,MET,MOP,MTD,THC
the expiration date.
Cont.: 30 panels, individually packed (30x REF Z13960B)
Cont.: 1 panel, individually packed
SAMPLE COLLECTION AND PREPARATION
Multi-6/7 Drug Panel BUP,BZO,COC,MOP,MTD,THC
The urine must be collected in a clean and dry container. Urine collected at any time of the
Cont.: 30 panels, individually packed (30x REF Z09970B)
day may be used. Urine specimens exhibiting visible precipitations should be centrifuged,
Cont.: 1 panel, individually packed
filtered or allowed to settle to obtain a clear specimen for testing. Urine specimens may be stored at 2-8°C for up to 48 h prior to testing. For prolonged storage, specimens may be
Multi-6/10 Drug Panel AMP,BZO,COC,MOP,MTD,THC
Cont.: 30 panels, individually packed (30x REF Z11911B)
frozen and stored below –20°C. Frozen specimens should be thawed and mixed before
Cont.: 1 panel, individually packed
Multi-7 Drug Panel AMP,BUP,BZO,COC,MTD,MOP,THC
ASSAY PROCEDURE
Cont.: 30 panels, individually packed (30x REF Z12730B)
Allow the test panel, urine specimen, and/or controls to equilibrate to room tempera-
Cont.: 1 panel, individually packed
ture (15-30°C) prior to testing
Multi-10 Drug Panel AMP,BAR,BZO,COC,MDMA,MET,MOP,MTD,TCA,THC
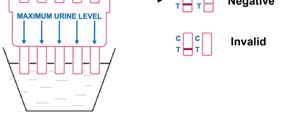
Remove the test panel from the sealed pouch and use it as soon as possible.
Cont.: 30 panels, individually packed (30x REF Z04230B)
Take off the protective cap plugged
Cont.: 10 panels, individually packed (10x REF Z04230B)
on the test panel. With arrows
Cont.: 1 panel, individually packed
pointing towards the urine
Multi-10/1 Drug Panel AMP,BAR,BZO,BUP,COC,MDMA,MET,MOP,MTD,THC
specimen, immerse the test panel
Cont.: 30 panels, individually packed (30x REF Z05235B)
vertically into the urine specimen
Cont.: 10 panels, individually packed (10x REF Z05235B)
for 10-15 seconds. Do not allow
Cont.: 1 panel, individually packed
the urine sample to touch the plastic cassette when immersing
Multi-10/2 Drug Panel AMP,BAR,BZO,COC,MDMA,MOP,MTD,OPI,TCA,THC
Cont.: 30 panels, individually packed (30x REF Z06102B)
the test device into the urine
Cont.: 1 panel, individually packed
sample. Avoid immersion of the cassette deeper than the mark
Multi-10/3 Drug Panel AMP, BZO,COC,MDMA,MOP,MTD,OPI,PCP,TCA,THC
indicated with the arrows on the
Cont.: 30 panels, individually packed (30x REF Z06103B)
device and avoid any direct contact
Cont.: 1 panel, individually packed
of the sample with the test region.
Multi-10/4 Drug Panel AMP,BAR,BUP,BZO,COC,MDMA,MET,MTD,OPI,THC
3. Put the protective cap back onto
Cont.: 30 panels, individually packed (30x REF Z06104B)
the test panel. Place the test panel
Cont.: 1 panel, individually packed
on a non-absorbent flat surface, start the timer and wait for the red line(s) to appear.
Multi-10/5 Drug Panel AMP,BAR,BZO,BUP,COC,MET,MOP,MTD,TCA,THC
Read the results at 5 minutes. Do not interpret results after 10 minutes.
Cont.: 30 panels, individually packed (30x REF Z06105B)
Cont.: 1 panel, individually packed
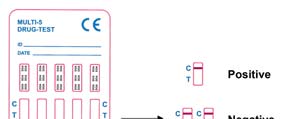
INTERPRETATION OF RESULTS
Multi-10/6 Drug Panel AMP,BAR,BZO,COC,MET,MOP,MTD,PCP,TCA,THC
NEGATIVE: A colored line in the control region (C) and a colored line in the test line
Cont.: 30 panels, individually packed (30x REF Z06106B)
region (T) for a specific drug indicate a negative results. This indicates that the drug
Cont.: 1 panel, individually packed
concentration in the urine specimen is below the designated cut-off level for that specific drug.
Multi-10/7 Drug Panel AMP,BAR,BZO,COC,MET,MTD,OPI,PCP,TCA,THC
Cont.: 30 panels, individually packed (30x REF Z06107B)
*NOTE: The shade of color in the test region (T) may vary, but it should be considered
Cont.: 1 panel, individually packed
negative whenever there is even a faint pink line.
All products contain a package insert!
POSITIVE: A colored line in the control line region (C) but no line in the test line region
(T) for a specific drug indicates a positive results. This indicates that the drug concentra-
For in vitro diagnostic use only. For use by medical professionals only.
tion in the urine specimen exceeds the designated cut-off level.
For diagnosis and therapeutic monitoring only.
INVALID: Control line fails to appear. Insufficient specimen volume or incorrect procedural
INTENDED USE
techniques are the most likely reasons for control line failure. Review the procedure and
The "DIAQUICK" Multi-Drug Panels (urine) are rapid, lateral flow chromatographic immunoas-
repeat the test using a new test panel. If the problem persists, discontinue using the lot
says for the simultaneous, qualitative detection of the fol owing drugs and their metabolites:
immediately and contact your local distributor
Parameter
Short Calibrator Substance
QUALITY CONTROL
Amphetamine
AMP d-Amphetamine
A procedural control is included in the test. A red line appearing in the control region (C) is
BAR Secobarbital
considered an internal procedural control. It confirms sufficient specimen volume, adequate
BUP Buprenorphine
membrane wicking and correct procedural technique. Control standards are not supplied with this kit; however, it is recommended that positive and negative controls be tested as good
laboratory practice to confirm the test procedure and to verify proper test performance.
COC Benzoylecgonine
LIMITATIONS
MDMA d,l Methylenedioxymethamphetamine
1. The "DIAQUICK" Multi-Drug Panels (urine) provide only a preliminary analytical result.
MET d-Methamphetamine
A more specific chemical method must be used to obtain a confirmed result. Gas chro-
Methadone
matography/mass spectrometry (GC/MS) is the preferred confirmatory method.
Opiate, Morphine, Heroine
2. It is possible that technical or procedural errors, as wel as other interfering substances
in the urine specimen may cause erroneous results.
Opiate, Morphine, Heroine
3. Adulterants, such as bleaching agents in urine specimens may produce erroneous
PCP Phencyclidine
results regardless of the analytical method used. If adulteration is suspected, the test
Tricyclic Antidepressants
TCA Nortriptyline
should be repeated with another urine specimen.
THC 11-nor-∆9-THC-9-COOH 50
A positive result indicates presence of the drug or its metabolites but does not indicate
Mag. Simone Sturm-E.

DIALAB Produktion und Vertrieb von chemisch – technischen Produkten und Laborinstrumenten Gesellschaft m.b.H.
A – 2351 Wiener Neudorf, Austria, IZ-NÖ Süd, Hondastrasse, Objekt M55
Phone: ++43 (0) 2236 660910-0, Fax: ++43 (0) 2236 660910-30, e-mail: [email protected]
the level of intoxication, administration route or concentration in urine.
ANALYTICAL SPECIFICITY
5. A negative result may not necessarily indicate drug-free urine. Negative results can be
The fol owing tables lists the concentration of compounds (ng/mL) that are detected positive
obtained if a drug is present but below the cut-off level of the test.
in urine by the "DIAQUICK" Multi-Drug Panels (urine) at 5 minutes.
6. The "DIAQUICK" Multi-Drug Panels (urine) do not distinguish between drugs of abuse
AMPHETAMINES (AMP)
AMP ECSTASY
and certain medications.
d,l-Amphetamine sulfate
200 (±) 3,4-Methylenedioxymethamphetamine HCl
7. A positive result might be obtained from certain foods or food supplements.
l-Amphetamine 25,000
(±) 3,4-Methylenedioxyamphetamine HCl (MDA)
PERFORMANCE CHARACTERISTICS
d-Amphetamine 1,000
3,4-Methylenedioxyethylamphetamine (MDE)
(±) 3,4-Methylenedioxyamphetamine
400 OPIATE, MORPHINE, HEROINE
ACCURACY
Maprotiline 50,000
A side-by-side comparison was conducted using the "DIAQUICK" Multi-Drug Panels (urine)
Methoxyphenamine 6,000
and commercial y available drug rapid tests. Testing was performed on approximately 250
specimens. Presumptive positive results were confirmed by GC/MS. The fol owing results
BAR Hydromorphone
% Agreement with Commercial Kit
3,000 6-Monoacethylmorphine
Positive Agreement
Negative Agreement
Total Results
300 Morphine 3-β-d-glucuronide 800
6,000 Norcodeine
Cyclopentobarbital
5,5-Diphenylhydantoin
6,000 MARIHUANA/CANNABIS
300 11-nor-∆8- THC-9 COOH
11-nor-∆9- THC-9 COOH
BZO ∆8- THC
a-hydroxyalprazolam
1,500 OPIATE, MORPHINE, HEROINE
* NOTE: Based on GC/MS instead of Commerical Kit.
% Agreement with GC/MS
Chlordiazepoxide
780 Ethylmorphine
Positive Agreement
Negative Agreement
Total Results
390 Hydromorphone
Clorazepate dipotassium
780 6-Monoacethylmorphine
Desalkylflurazepam
200 Morphine 3-β-d-glucuronide 2,000
6,250 Norcodeine
RS-Lorazepam glucuronide
Norchlordiazepoxide
100 PHENCYCLIDINE
780 Phencyclidine
300 4-Hydroxyphencyclidine
ANALYTICAL SENSITIVITY
100 TRICYCLIC ANTIDEPRESSANTS
A drug-free urine pool was spiked with drugs to the concentrations at ± 50% cut-off and ±
3,100 Amitriptyline
25% cut-off. The results are summarized below:
BUP Clomipramine 50,000
Cyclobenzaprine
AMP
BAR BUP BZO COC
Drug Conc.
n
Buprenorphine 3-d-Glucuronide
Norbuprenorphine
-50% Cut-off
3-d-Glucuronide 100
-25% Cut-off
COC Maprotiline 1,500
Benzoylecgonine 300
+25% Cut-off
200 Nortriptyline
+50% Cut-off
Cocaethylene 12,500
30,000 Promazine
MTD
MET
MDMA MOP
OPI
METHADONE
MTD Promethazine 25,000
Drug Conc.
Doxylamine 100,000
-50% Cut-off
-25% Cut-off
d-Methamphetamine
+25% Cut-off
l-Methamphetamine 12,500
+50% Cut-off
Mephentermine 50,000
PCP
TCA
THC
Drug Conc.
REFERENCES
-50% Cut-off
Hawks RL, CN Chiang. Urine Testing for Drugs of Abuse. National Institute for Drug Abuse
-25% Cut-off
(NIDA), Research Monograph 73, 1986.
Tietz NW. Textbook of Clinical Chemistry. W.B. Saunders Company. 1986; 1735.
+25% Cut-off
Stewart DJ, Inaba T, Lucassen M, Kalow W. Clin. Pharmacol. Ther. April 1979; 25 ed: 464,
+50% Cut-off
Ambre J. J. Anal. Toxicol.1985; 9:241.
Winger, Gail, A Handbook of Drug and Alcohol Abuse, Third Edition, Oxford Press, 1992,
A study was conducted to determine the cross-reactivity of the test with compounds in either
Robert DeCresce. Drug Testing in the workplace, 1989 page 114.
drug-free urine or drug positive urine. The fol owing compounds did not show a cross-
Glass, IB. The International Handbook of Addiction Behavior. Routledge Publishing, New
reactivity when tested with the "DIAQUICK" Multi-Drug Panels (urine) at a concentration of
York, NY. 1991; 216
B. Cody, J.T., "Specimen Adulteration in drug urinalysis. Forensic Sci. Rev., 1990, 2:63.
Non Cross-Reacting Compounds:
C. Tsai, S.C. et.al., J. Anal. Toxicol. 1998; 22 (6): 474
Acetophenetidin Cortisone
d-Pseudoephedrine 10. Baselt RC. Disposition of Toxic Drugs and Chemicals in Man. 6th Ed. Biomedical Publ.,
N-Acetylprocainamide Creatinine
Foster City, CA 2002.
Acetylsalicylic acid
Deoxycorticosterone
11. Hardman JG, Limbird LE. Goodman and Gilman's: The Pharmacological Basis for Thera-
Aminopyrine Dextromethorphan
Loperamide Salicylic
peutics. 10th Edition. McGraw Hill Medical Publishing, 2001; 208-209.
Amoxicillin Diclofenac Meprobamate
Ampicillin Diflunisal Methoxyphenamine
Apomorphine Diphenhydramine
acid Tetracycline
Aspartame Ethyl-p-aminobenzoate
Naproxen Tetrahydrocortisone,
β-Estradiol Niacinamide
Estrone-3-sulfate
Tetrahydrocortisone
Tetrahydrozoline
Bilirubin Fenoprofen
d,l-Brompheniramine Furosemide
Caffeine Gentisicacid
Cannabidiol Hemoglobin Oxolinic
acid Tolbutamide
Chloralhydrate Hydralazine
Oxymetazoline Triamterene
Chloramphenicol Hydrochlorothiazide Papaverine
Chlorothiazide Hydrocortisone Penicillin-G
d,l-Chlorpheniramine o-Hydroxyhippuric
acid Perphenazine
Chlorpromazine 3-Hydroxytyramine
Cholesterol d,l-Isoproterenol
Prednisone Verapamil
Clonidine Isoxsuprine
Mag. Simone Sturm-E.

DIALAB Produktion und Vertrieb von chemisch – technischen Produkten und Laborinstrumenten Gesellschaft m.b.H.
A – 2351 Wiener Neudorf, Austria, IZ-NÖ Süd, Hondastrasse, Objekt M55
Phone: ++43 (0) 2236 660910-0, Fax: ++43 (0) 2236 660910-30, e-mail: [email protected]
"DIAQUICK" Multi-Drug Paneler
Denne testen vil oppdage andre beslektede forbindelser, vennligst se tabellen Analytisk spesifisitet i dette pakningsvedlegget. Denne analysen gir kun et foreløpig analytisk resultat.
for humane urinprøver
En mer spesifikk alternativ kjemisk fremgangsmåte må brukes for å få et bekreftet analytisk resultat. Gasskromatografi/massespektrometri (GC/MS) er den foretrukne bekreftende
Multi-3 Drug Panel BZO,COC,MOP
metode. Klinisk vurdering og profesjonelt skjønn skal anvendes for alle testresultater relatert
Innhold: 30 Paneler, individuelt pakket (30x REF Z06576B)
til misbruksanalyser, særlig når de foreløpige resultatene er positive. Kun til in vitro diagnos-
Innhold: 1 Panel, individuelt pakket
Multi-3/1 Drug Panel
Innhold: 30 Paneler, individuelt pakket (30x REF Z09577B)
TEST PRINSIPP
Innhold: 1 Panel, individuelt pakket
"DIAQUICK" Multi-Drug Panels (urin) er immunanalyser basert på prinsippet om konkurrer-ende binding. Narkotika som kan være tilstede i urinprøven konkurrerer mot deres respektive
Multi-4 Drug Panel AMP,COC,MOP,THC
Innhold: 30 Paneler, individuelt pakket (30x REF Z02575B)
narkotiske konjugat for bindingssteder på deres spesifikke antistoff. Under testing, vandrer
Innhold: 1 Panel, individuelt pakket
urinprøven oppover med kapillæreffekt. Narkotika, hvis tilstede i urinprøven under cut-off konsentrasjon, vil det ikke mette bindingsstedene for sitt spesifikke antistoff belag på
Multi-5 Drug Panel BZO,COC,MET,MOP,THC
partiklene. Antistoffbelagte partikler vil da bli fanget opp av det immobiliserte stoffkonjugatet
Innhold: 30 Paneler, individuelt pakket (30x REF Z05236B)
og en synlig farget linje vil dukke opp i testlinjeområdet på teststrimmelen for det spesifikke
Innhold: 1 Panel, individuelt pakket
stoffet. Det dannes ingen farget linje i testlinjeområdet hvis stoffnivået er over cut-off
Multi-5/3 Drug Panel AMP,COC,MET,MOP,THC
konsentrasjonen, fordi de metter alle bindingsstedene til antistoffet belagt på partiklene. En
Innhold: 30 Paneler, individuelt pakket (30x REF Z06502B)
urinprøve som tester positivt for narkotika, vil ikke generere en farget linje i det spesifikke
Innhold: 1 Panel, individuelt pakket
testlinjeområdet på teststrimmelen på grunn av konkurrerende stoffer, men en urinprøve som
Multi-5/4 Drug Panel AMP,COC,MDMA,MOP,THC
tester negativt for narkotika eller en prøve som inneholder en stoffkonsentrasjon som er
Innhold: 30 Paneler, individuelt pakket (30x REF Z11504B)
lavere enn cut-off verdien, vil generere en linje i testlinjeområdet. For å fungere som en
Innhold: 1 Panel, individuelt pakket
prosedyrekontroll, vil det alltid vises en farget linje i kontrollinjeområdet, som indikerer at riktig prøvevolum er tilsatt og at fukttranssport på membranen har foregått.
Multi-5/6 Drug Panel AMP,BZO,COC,MOP,THC
Innhold: 30 Paneler, individuelt pakket (30x REF Z06506B)
ADVARSLER OG FORHOLDSREGLER
Innhold: 1 Panel, individuelt pakket
Kun for medisinsk og annen in vitro diagnostisk bruk. Ikke bruk etter utløpsdatoen.
Multi-6 Drug Panel BZO,COC,MET,MOP,MTD,THC
Testpanelet må oppbevares i den forseglede posen til det skal brukes.
Innhold: 30 Paneler, individuelt pakket (30x REF Z98907B)
Alle prøver skal betraktes som potensielt farlige og håndteres på samme måte som
Innhold: 1 Panel, individuelt pakket
smittefarlige materialer.
Multi-6/1 Drug Panel AMP,BZO,COC,MET,MOP,THC
Brukte testpaneler skal kasseres i henhold til statlige og lokale forskrifter
Innhold: 30 Paneler, individuelt pakket (30x REF Z03220B)
REAGENSER
Innhold: 1 Panel, individuelt pakket
Hver testlinje inneholder anti-narkotika monoklonalt antistoff fra mus og tilhørende protein-
Multi-6/3 Drug Panel
BUP, BZO, COC, MTD, OPI, THC
konjugater fra denne narkotikaen. Kontrollinjen inneholder geit anti-kanin-IgG polyklonale
Innhold: 30 Paneler, individuelt pakket (30x REF Z08930B)
antistoffer og kanin IgG.
Innhold: 1 Panel, individuelt pakket
OPPBEVARING
Multi-6/4 Drug Panel AMP,BUP,BZO,MET,MOP,THC
Innhold: 30 Paneler, individuelt pakket (30x REF Z08940B)
"DIAQUICK" Multi-Drug Panelene kan oppbevares i kjøleskap eller ved romtemperatur (2-30
Innhold: 1 Panel, individuelt pakket
°C). Testpanelet er stabilt til og med utløpsdatoen som er trykt på den forseglede posen. Testpanelet må oppbevares i den forseglede posen til det skal brukes. MÅ IKKE FRYSES.
Multi-6/6 Drug Panel BUP,COC,MET,MOP,MTD,THC
Ikke bruk etter utløpsdatoen.
Innhold: 30 Paneler, individuelt pakket (30x REF Z13960B)
Innhold: 1 Panel, individuelt pakket
PRØVETAKING OG KLARGJØRING
Multi-6/7 Drug Panel BUP,BZO,COC,MOP,MTD,THC
Urinprøven må innhentes i en ren og tørr beholder. Urin samlet inn når som helst på dagen
Innhold: 30 Paneler, individuelt pakket (30x REF Z09970B)
kan anvendes. Urinprøver med synlig utfelling bør sentrifugeres, filtreres eller få bunnfalle
Innhold: 1 Panel, individuelt pakket
seg for å få klar prøve for testing. Urinprøver kan lagres ved 2-8°C i opptil 48 timer før analysering. For langvarig lagring, kan prøvene fryses og lagres under -20 °C. Frosne prøver
Multi-6/10 Drug Panel AMP,BZO,COC,MOP,MTD,THC
Innhold: 30 Paneler, individuelt pakket (30x REF Z11911B)
må tines og blandes før testing.
Innhold: 1 Panel, individuelt pakket
Multi-7 Drug Panel AMP,BUP,BZO,COC,MTD,MOP,THC
La testpanelet, urinprøven, og/eller kontrollene oppnå romtemperatur (15-30 °C) før
Innhold: 30 Paneler, individuelt pakket (30x REF Z12730B)
testing.
Innhold: 1 Panel, individuelt pakket
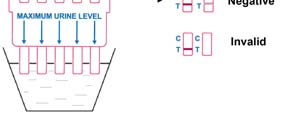
Ta testpanelet ut av den forseglede posen og bruk det så snart som mulig.
Multi-10 Drug Panel AMP,BAR,BZO,COC,MDMA,MET,MOP,MTD,TCA,THC
2. Ta av beskyttelseshetten som er
Innhold: 30 Paneler, individuelt pakket (30x REF Z04230B)
plugget på testpanelet. Med pilene
Innhold: 10 Paneler, individuelt pakket (10x REF Z04230B)
pekende mot urinprøven, dypp
Innhold: 1 Panel, individuelt pakket
testpanelet vertikalt i urinprøven for
Multi-10/1 Drug Panel AMP,BAR,BZO,BUP,COC,MDMA,MET,MOP,MTD,THC
10-15 sekunder. Ikke la urinprøven
Innhold: 30 Paneler, individuelt pakket (30x REF Z05235B)
komme i kontakt med plastkassetten
Innhold: 10 Paneler, individuelt pakket (10x REF Z05235B)
mens testenheten dyppes i
Innhold: 1 panel, individually packed
urinprøven. Unngå nedsenkning av kassetten dypere enn merket med
Multi-10/2 Drug Panel AMP,BAR,BZO,COC,MDMA,MOP,MTD,OPI,TCA,THC
Innhold: 30 Paneler, individuelt pakket (30x REF Z06102B)
pilene på enheten, og unngå at
Innhold: 1 Panel, individuelt pakket
testområdet kommer direkte i kontakt med prøven.
Multi-10/3 Drug Panel AMP,
Innhold: 30 Paneler, individuelt pakket (30x REF Z06103B)
3. Sett beskyttelseshetten tilbake på
Innhold: 1 Panel, individuelt pakket
testpanelet. Plasser testpanelet på et ikke-absorberende flatt underlag,
Multi-10/4 Drug Panel AMP,BAR,BUP,BZO,COC,MDMA,MET,MTD,OPI,THC
start tidtakeren og vent på at den
Innhold: 30 Paneler, individuelt pakket (30x REF Z06104B)
(de) røde linjen(e) skal vises. Avles
Innhold: 1 Panel, individuelt pakket
resultatene etter 5 minutter. Ikke tolk resultater etter 10 minutter.
Multi-10/5 Drug Panel AMP,BAR,BZO,BUP,COC,MET,MOP,MTD,TCA,THC
Innhold: 30 panels, individually packed (30x REF Z06105B)
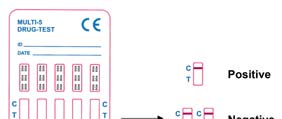
TOLKNING AV RESULTATER
Innhold: 1 Panel, individuelt pakket
NEGATIV: En farget linje i kontrollinjeområdet (C) og en farget linje i testlinjeområdet
Multi-10/6 Drug Panel AMP,BAR,BZO,COC,MET,MOP,MTD,PCP,TCA,THC
(T) for et bestemt narkotisk stoff indikerer et negativt resultat. Dette indikerer at
Innhold: 30 Paneler, individuelt pakket (30x REF Z06106B)
konsentrasjonen for narkotiske stoffet i urinprøven er under angitt cut- off nivå for dette
Innhold: 1 Panel, individuelt pakket
bestemte narkotika.
Multi-10/7 Drug Panel AMP,BAR,BZO,COC,MET,MTD,OPI,PCP,TCA,THC
*MERK: Fargenyansen i testlinjeområdet (T) kan variere, men resultatet skal anses som
Innhold: 30 Paneler, individuelt pakket (30x REF Z06107B)
negativet selv om det bare vises en svak rosa linje.
Innhold: 1 Panel, individuelt pakket
POSITIV: En farget linje i kontrollinjeområdet (C), men ingen linje i testlinjeområdet (T)
Alle produktene inneholder et pakningsvedlegg!
for et bestemt narkotika indikerer et positivt resultat. Dette indikerer at konsentrasjonen
Kun til in vitro diagnostisk bruk. Skal kun brukes av medisinsk fagpersonell. Kun til
av narkotikaen i urinprøven overstiger det angitte cut-off nivået.
diagnostikk og terapeutisk overvåkning.
UGYLDIG: Kontrollinjen vises ikke. Utilstrekkelig prøvevolum eller feil prosedyreteknikk er
TILTENKT BRUK
de mest sannsynlige årsakene til manglende kontrollinje. Gå gjennom prosedyren og gjenta
"DIAQUICK" Multi-Drug Paneler (urin) er raske, lateral flytkromatografiske immunanalyser for
testen med et nytt testpanel. Hvis problemet vedvarer, slutt å bruke loten umiddelbart og
samtidig, kvalitativ påvisning av følgende narkotiske stoffer og deres metabolitter:
kontakt din lokale leverandør.
Parameter
Kort Kalibrator
Amfetamin
AMP d-Amphetamin
En intern prosedyrekontroll er inkludert i testen. Den røde linjen som vises i kontrollinjeo-mrådet (C), er en intern prosedyrekontrol . Det bekrefter tilstrekkelig prøvevolum, tilstrekkelig
BAR Secobarbital
fukttransportering på membranen og riktig prosedyreteknikk. Kontrol er følger ikke med dette
Buprenorfin
kitet. Det anbefales imidlertid at positive og negative kontrol er testes for god laboratori-
epraksis, for å bekrefte testprosedyren og til å verifisere riktig testytelse.
COC Benzoylecgonin
MDMA d,l Metylenedioxymetamfetamin
1. "DIAQUICK" Multi-Drug Paneler (urin) gir kun et foreløpig analytisk resultat. En mer
MET d-Metamfetamin
spesifikk kjemisk metode må brukes for å få bekreftet resultatet. Gasskromatogra-
fi/massespektrometri (GC/GS) en den foretrukne bekreftende metode.
2. Det er mulig at tekniske eller prosedyrefeil, samt andre forstyrrende stoffer i urinprøven
Opiat, Morfin, Heroin
kan gi feilaktige resultater.
Opiat, Morfin, Heroin
3. Forfalskningsmidler, slik som blekemiddel i urinprøver, kan gi feilaktige resultater uavhen-
Fensyklidin
gig av den analytiske metoden som brukes. Hvis forfalskning mistenkes, bør testen gjen-tas med en annen urinprøve.
Trisykliske antidepressiva
TCA Nortriptylin
4. Et positivt resultat indikerer tilstedeværelse av det narkotiske stoffet eller dets metabolitter,
Marihuana/Cannabis
THC 11-nor-∆9-THC-9-COOH 50
men angir ikke nivået på rus, administrasjonsvei eller konsentrasjon i urin.
Mag. Simone Sturm-E.

DIALAB Produktion und Vertrieb von chemisch – technischen Produkten und Laborinstrumenten Gesellschaft m.b.H.
A – 2351 Wiener Neudorf, Austria, IZ-NÖ Süd, Hondastrasse, Objekt M55
Phone: ++43 (0) 2236 660910-0, Fax: ++43 (0) 2236 660910-30, e-mail: [email protected]
5. Et negativt resultat indikerer nødvendigvis at urinen ikke inneholder narkotika. Negative
resultater kan oppnås dersom narkotika er tilstede, men under cut-off nivået av testen.
(±) 3,4-Metylendioxymetamfetamin HCl
6. "DIAQUICK" Multi-Drug Panels (urin) skiller ikke mellom narkotika misbruk og enkelte
25 000 (±) 3,4-Metylendioxyamfetamin HCl
(±) 3,4-Metylendioxyetylmetamftamin
7. Et positivt resultat kan oppnås fra visse matvarer eller kosttilskudd.
OPIAT, MORFIN, HEROIN
50 000 Kodein 200
YTELSE OG EGENSKAPER
Hydrokodon 50 000
En side-ved-side-sammenligning ble gjennomført ved hjelp av "DIAQUICK" Multi-Drug Paneler
Hydromorfon 3 000
(urin) og kommersielt tilgjengelige hurtigtester for påvisning av narkotika. Testene ble utført på
250 prøver. Presumptive positive resultater ble bekreftet ved GC/MS. Følgende resultater ble
6-Monacetylmorfin 400
Morfin 3-β-d-glukuronid
% samsvar med kommersielt kit
Positivt samsvar
Negativt samsvar
Totalt samsvar
Oksymorfon 50 000
25 000 Prokain 15 000
Cannabinol 20 000
11-nor-∆8- THC-9 COOH
11-nor-∆9- THC-9 COOH
a-hydroxyalprazolam 1 500
OPIAT, MORFIN, HEROIN
Hydrokodon 50 000
* MERK: Basert på GC/MS i stedet for kommersielt kit.
Hydromorfon 12 500
% Übereinstimmung mit GC/MS
Levorfanol 25 000
6-Monacetylmorfin 3 000
Positivt samsvar
Negativt samsvar
Totalt samsvar
Morfin 3-β-d-glukuronid
Oksymorfon 25 000
Norklordiazepoksid 100
FENSYKLIDIN (PCP)
4-Hydroxyfensyklidin 6 250
TRISYKLISKE ANTIDEPRESSIVA
Amitriptylin 1 500
Klomipramin 50 000
Sykobenzaprin 1 500
ANALYTISK SENSITIVITET
En urinpool fri for narkotika ble tilsatt narkotikum med cut off verdier på ± 50% og ± 25% .
Resultatene oppsummeres nedenfor:
Matprotilin 1 500
AMP
BAR BUP BZO COC
n
Nortriptylin 1 000
12 500 Perfenazin 25 000
30 000 Promazin 3 000
Prometazin 25 000
Trimipramin 3 000
MTD
MET
MDMA MOP
OPI
(±) 3,4-Metylendioxymetamfetamin
REFERANSER
Hawks RL, CN Chiang. Urine Testing for Drugs of Abuse. National Institute for Drug Abuse
PCP
TCA
THC
(NIDA), Research Monograph 73, 1986.
Tietz NW. Textbook of Clinical Chemistry. W.B. Saunders Company. 1986; 1735.
Stewart DJ, Inaba T, Lucassen M, Kalow W. Clin. Pharmacol. Ther. April 1979; 25 ed: 464,
Ambre J. J. Anal. Toxicol.1985; 9:241.
Winger, Gail, A Handbook of Drug and Alcohol Abuse, Third Edition, Oxford Press, 1992,
Robert DeCresce. Drug Testing in the workplace, 1989 page 114.
Det ble gjennomført en studie for å bestemme testens kryssreaktivitet med forbindelse i
Glass, IB. The International Handbook of Addiction Behavior. Routledge Publishing, New
enten narkotikafri urin eller narkotikapositiv urin. Følgende forbindelser viste ingen kryssre-
York, NY. 1991; 216
aktivitet når testet med "DIAQUICK" Multi-Drug Paneler (urin) i en konsentrasjon på 100
B. Cody, J.T., "Specimen Adulteration in drug urinalysis. Forensic Sci. Rev., 1990, 2:63.
C. Tsai, S.C. et.al., J. Anal. Toxicol. 1998; 22 (6): 474
10. Baselt RC. Disposition of Toxic Drugs and Chemicals in Man. 6th Ed. Biomedical Publ.,
Forbindelser uten kryssreaktivitet:
Foster City, CA 2002.
Acetophenetidin Cortison
d-Pseudoephedrin
11. Hardman JG, Limbird LE. Goodman and Gilman's: The Pharmacological Basis for Thera-
N-Acetylprocainamid Creatinin
peutics. 10th Edition. McGraw Hill Medical Publishing, 2001; 208-209.
Acetylsalicylsäure Deoxycorticosteron
Aminopyrin Dextromethorphan
Loperamid Salicylsäure
Amoxicillin Diclofenac Meprobamat
Ampicillin Diflunisal Methoxyphenamin
l-Ascorbinsäure Digoxin
Methylphenidat Sulindac
Apomorphin Diphenhydramin
Aspartam Ethyl-p-aminobenzoat
Naproxen Tetrahydrocortison
β-Estradiol Niacinamid 3-acetat
Benzilinäsure Estron-3-sulfat Nifedipin
Tetrahydrocortison
Benzoesäure Erythromycin Norethindron Tetrahydrozolin
Bilirubin Fenoprofen
d,l-Brompheniramin Furosemid
Koffein Gentisinsäure
Cannabidiol Hämoglobin Oxolinsäure Tolbutamid Chloralhydrat Hydralazin
Oxymetazolin Triamteren
Chloramphenicol Hydrochlorothiazid
Chlorothiazid Hydrocortison Penicillin-G Trimethoprim d,l-Chlorpheniramin o-Hydroxyhippursäure
Chlorpromazin 3-Hydroxytyramin
Cholesterol d,l-Isoproterenol
Prednison Verapamil
Clonidin Isoxsuprin
ANALYTISK SPESIFISITET
Tabellene nedenfor viser konsentrasjonen av stoffer (ng/mL) som blir funnet positive i urin av
"DIAQUICK" Multi-Drug Panels (urin) på 5 minutter.
Mag. Simone Sturm-E.
Source: http://www.medic24.no/wp-content/uploads/2014/12/DOA-Multi-Panels-Rev17-en_no.pdf
3mpl.univ-angers.fr
Effect of Particle Morphology and Interaction on the Stabilization of Water/Water Emulsions by Protein Particles Alberto GONZALEZ-JORDAN, Taco NICOLAI, Lazhar BENYAHIA [email protected] Université du Maine, IMMM UMR CNRS 6283, PCI, 72085 Le Mans Cedex Category : Molécules Matériaux Water/water (W/W) emulsions have a promising potential for many applications, especially in the food and cosmetics industries. These emulsions cannot be stabilized by surfactants, but in recent years it was found that they can be stabilized by adding solid particles in a manner similar to so-called Pickering oil/water emulsions. Protein particles in the form of microgels, fractal aggregates or fibrils were made from the same protein, ß-lactoglobulin by heating at different conditions. The effect of the morphology on the stability and structure of W/W emulsions made by mixing aqueous solutions of PEO and dextran was investigated at different pH. Interestingly, excess proteins partioned to the dextran phase at pH>4 and to the PEO at pH<3.0. Fibrils were found to be most effective stabilizers at pH 7, whereas fractals were most effective at pH 3. At pH values between 5.5 and 3.5 the protein particles aggregated leading to cold gelation. If excess proteins were in the continuous phase W/W emulsion formed gels that were very weak, but strong enough to prevent creaming of sedimentation of the dispersed droplets. If excess proteins were in the dispersed phase, the droplets were transformed into stable microscopic protein particles.
Op-jeen150272 1.8
Journal of Economic Entomology Advance Access published September 5, 2015 Spray Toxicity and Risk Potential of 42 Commonly Used Formulations of Row Crop Pesticides to Adult Honey Bees (Hymenoptera: Apidae) YU CHENG ZHU,1,2 JOHN ADAMCZYK,3 THOMAS RINDERER,4 JIANXIU YAO,1 ROBERT DANKA,4 RANDALL LUTTRELL,1 AND JEFF GORE5 J. Econ. Entomol. 1–8 (2015); DOI: 10.1093/jee/tov269