About division of pharmaceuticals in course of blistering and it's permissibility
About division of pharmaceuticals in course of blistering and it´s EVALUATION ACCORDING TO THE ORDINANCE ON THE OPERATION OF PHARMACIES DR. RER. NAT. THOMAS WELLENHOFER AND RA VEITH RÖSSGER TRANSLATED BY SEBASTIAN BECK IfpiV Zwieselstr. 15, 83395 Freilassing Are divided pharmaceuticals in nursing homes replaceable? Is it necessary to proof drugs durability in case of division? In section 34 (1) No. 3 the Ordinance on the Operation of Pharmacies (Apothekenbetriebsordnung ApBetrO) requires a quality system, where pharmacies have to define "exceptional cases [in which] a written request by a physician may be fulfil ed regarding the division of tablets before dosage provision or blistering, if otherwise pharmaceutical care cannot be ensured and there is proven validation of stable quality over the shelf life of the blister or reusable container, even though subsequent alterations of the ready-made pharmaceutical should be prevented in principle." These political specifications meet expert's demand of former times.,The feedbacks to that new arrangements are versatile. Hence the National Union of Patient-individual Blistering (Bundesverband Patientenindividueller Arzneimittelverblisterer BPAV) sees manual division of tablets only immediately before application and under disposal of fragments as pharmaceutically correct.The physician´s newspaper is a bit stricter. There authors think that either physicians disclaim on division of pharmaceuticals or nursing homes on blistering.These statements are only less helpful in the daily routine. However it´s the papers goal to deal with a pharmaceutical correct and law conforming division of certain pharmaceuticals in supply of patients. 1 RIESENBERGER, 2007 2 SCHMIDT et. al, 2008 4 Physician´s newspaper, 16.4.2012 Juristical y demand The Ordinance on the Operation of Pharmacies neither prohibits division nor defines criteria, in which it is allowed. Basically permissibility of division of pharmaceuticals As mentioned in the beginning section 34 (1) No. 3 ApBetrO shows no specifications on division of pharmaceutics, but requires system rules for tablet division in exceptional cases of dosage provision or blistering according to quality management. Hence question of permissibility without special rules is occurring. Basically such a permissibility should be given: It is necessary to place criteria about tablet division in pharmacies quality management system, as mentioned in section 34 (1) No. 3 ApBetrO, only if decision opportunities towards divisibility are occurring. In case of criteria fulfillment, division is allowed. In pursuance of section 34 (1) No. 3 this conclusion is confirmed. It´s the legislative authorities will in general not to modify ready-made pharmaceuticals while dosage provision or blistering. Hence modification, such like division, of ready-made pharmaceuticals in exceptional cases is permitted. However the Lower House of German Parliament declares in an official statement to the Fourth Amendment of the Ordinance on the Operation of Pharmacies from 5th July 2012 in section 34 (1) No. 3 ApBetrO: "Die Entscheidung (Nummer3), ob Tabletten gegebenenfal s geteilt werden dürfen, ist von verschiednenen Faktoren abhängig zu machen. Eine Teilung von Tabletten kann nur infrage kommen, wenn eine tatsächliche Bruchril e (und nicht etwa eine Schmuckkerbe) vorhanden ist und diese laut Angabe in der Packungsbeilage einer Teilbarkeit im Sinne der Dosierung dient. Dabei ist zu berücksichtigen, dass sich die Angaben des pharmazeutischen Unternehmers zur Teilbarkeit nur auf die unmittelbare Einnahme nach der Teilung beziehen und keine Qualitätsaussagen zur Dauer einer möglichen Zwischenlagerung (u.a. auch im Blister) der geteilten Tabletten enthalten. Insofern muss diese in der Apotheke erfolgen. Im Übrigen sol te die Entscheidung, ob Tabletten geteilt werden dürfen, auch davon abhängig gemacht werden, ob die gewünschte Dosierung als Fertigarzneimittel nicht bereits verfügbar ist." Consequently the legislative authorities explicitly act on the assumption of a permissible tablet division. Requirements on permissible division of pharmaceuticals As already mentioned above, certain requirements on the division of pharmaceuticals are not defined in the Ordinance on the Operation of Pharmacies. But pharmacists are allowed to decide on an exceptional tablet division, whose fundamental frame is given by quality management, if the requirements are fulfilled. In this context it is necessary to consider that section 34 (1) No. 3 only entitles requirements in which pharmacists are allowed to define rules for division of tablets in course of quality management system. These requirements are: • Written request about division by a physician • Otherwise pharmaceutical care not available • Divided pharmaceutical with proven validation of stable quality through the shelf life of the delivery container Are divided pharmaceuticals in nursing homes replaceable? Now each requirement should be topic of the fol owing part. a) Demand of written physician request about division is clear. b) "Otherwise pharmaceutical care" means availability of analogous primary substance in the exhibited dose and galenics isn´t disposable. If there is a comparable delivery system of the same active
pharmaceutical ingredient with equal properties allocable, it will be obligatory to use it, referred to
the new ApBetrO. Division would be prohibited.
c) Without any relativization it is required that pharmaceutical quality must be assured for divided tablets over shelf life of the container. The manufacturers warrantee on the undivided drug is the legislators standard for the divided one. It´s the pharmacies task to prove this "validation". For finding a legal definition of "validation" search leads to the AMWHV section 2 (16) as well as the ApBetrO section 2 (16), where the fol owing is written down: "Validation is the provision of documented evidence which proves with high certainty that a pharmaceutical is prepared and tested based on a specific process or a standard operating procedure that meets the previously determined quality requirements." Apart from clearing the meaning of the term "validation", there is occurring the question of what has to be validated. The Ordinance on the Operation of Pharmacies offers no great help by answering this question. Obviously legislature was uncertain himself. Clues or advices with regard to the burden of proof are not perceptible. Rather Upper and Lower House of German Parliament declare in their laws amendment that pharmacists are not getting enough data about the influence of tablet division by the manufacturers. For lack of practical regulations we have to narrow requirements on validation down. The phrase "stable quality" probably intends criteria, which has to be fulfilled before reaching date of expiry. These requirements must be obeyed over administration of pharmaceuticals ("over the shelf life of the blister or reusable container"). Hence there are various requirements, if an inevitable division is carried out: • Dosage uniformity • Physical and chemical shelf life of the pharmaceutical form • Preservation of release properties Is at least one criterion missing or it isn´t possible to generate the required evidence, division is prohibited. With regard to existing uncertainty according to the requirements on validation, the pharmacist is left in the dark about achieving legally "proven validation of stable quality". Conclusion In pharmacists all-day routine remain unanswered questions referring to supply of patients by divided pharmaceuticals in consideration of the new ApBetrO. This ordinance although hits division of pharmaceuticals, but a clear handling is missing. According to section 34 (1) No. 3 three essential requirements are given, which put the pharmacist in the position to make a decision about the permissibility of tablet division in exceptional case to deliver a physician's request. These requirements are written physician´s request about division, no otherwise pharmaceutical care available and proven validation of stable quality. 6 ApBetrO section 34 (1) No. 3 Are divided pharmaceuticals in nursing homes replaceable? Especially the obviously wanted essential requirement of "proven validation of stable quality over the shelf life of the blister or reusable container" offers no concrete procedure, how to achieve "proven validation". From this results an uncertainty becomes the pharmacist's burden, who has to decide whether a request should be supplied or not. Therefore section 34 (1) No. 3 ApBetrO affects the physician´s right to prescribe certain pharmaceuticals on a secondary way. Conflicts in this context are predetermined. Besides it´s not defined which good of law attaches the higher value. It remains to be seen, if legislative authorities, jurisdiction or practical experience are bringing clarification. Currently all involved persons should focus on solving discrepancy with respect to the best supply of the affected patients. Pharmaceutical contemplation According to a circular letter of the Regional Chamber of Pharmacists for Bavaria from March 2013 turned up quite a lot of pharmaceutical evaluations to the discrepancy the following passage should deal with. Alternatives for pharmaceutical care Real alternatives of the required primary substance are offered in most of the cases only by liquid pharmaceuticals, because otherwise division wouldn´t be necessary. In comparison to solid compacts, liquids can be divided more easily because of their nature. A great additional expenditure makes other dosage forms hardly suitable (parenterals). Without pharmacokinetic similarity as well are dermal applications and inhalers. Additionally such dosage forms usually lead to considerable increase in price. On closer examination it attends attraction, that liquid dosage forms (drops, suspension or succus; sprays aren´t measureable) are often not available, see Besides an equal pharmaceutical supply is not possible, because of the differences in drug release and resorption between tablets and liquids. Quality assurance of divided pharmaceuticals With respect to pharmacist's way of view, there is only one formulation of a question. Does deviation influence quality of individual pharmaceuticals and - in case of agreement, is the modification in quality important or not? Besides it is necessary to design standard operation procedure, in which quality can be assured. Decision regarding deviation of each pharmaceutical and the consequences for liberation kinetics, for instance destroying of coating, is required, too. With help of investigations on shelf life it is possible to frame an answer on that question. Accuracy of dosage European pharmacopeia offers binding guideline according dosage accuracy of deviated pharmaceuticals. To assure intended dose, deviated drugs have to fulfill the test "Uniformity of mass". It is Pharmacopeias request that each permitted dose must be proven. Hence each pharmaceutical wanted to be divisible must achieve that test for authorization by legislature, which is a quite comprehensible demand according to known problems of deviation processes. Dosage form The solid oral long-term medication is effected by the regulation. According to the before mentioned question they can classified into two groups: • Coated pharmaceuticals, including Ocas-systems • Conventional tablets, MUPS-formulations and uncoated matrix systems 8 SCHÖFFLING, 2009 9 Ph. Eur. 7.0, 2.9.5, 2011 10 QUINZLER, 2006 and VERRUE 2011 Are divided pharmaceuticals in nursing homes replaceable? Coated pharmaceuticals There are several reasons for coating solid pharmaceuticals, like taste, mucosa protection and release kinetics. It is necessary to act on the assumption, that coating influences liberation, except the manufacturer publishes another statement. Normal y coating decreases degradation, because capsule coating, film or drageé shell controls diffusion of potential reactants like oxygen- and water molecules. In addition it inhibits light. Coating destruction by breakage changes reaction behavior completely. That´s why permissibility of division must be investigated in detail according to legitimate demand. Uncoated pharmaceuticals (tablets) But tablets are showing a totally different situation. With the exception of surface enlargement, these uncoated, monolithic compacts getting no changes, for instance in permeability or contact to reactants. Hence the fol owing part deals with the surface extension in case of bisection, which is the more common and better studied way of division.
Change in surface Via surface enlargement division of pharmaceuticals maybe lead to acceleration of degradation processes. Tablet´s surface depends on its shape, which is very versatile. Alongside the conventional cylindrical tablets (beveled as well as curved) there are existing different types like oval, three-/ four-sided, hearted or rod-shape.In huge majority of uncoated ones are cylindrical, oval or rod-shaped. Provided that bisection occurs along it´s breaking notch respectively with the objective of easy dividing, the following formula can be developed: Under breaking a cylindrical tablet, its surface increases for coefficient diameter x height x 2. Hence the following formula was established to describe ratio of total surface: 2 × 𝑆(𝑑𝑑𝑑𝑑𝑑𝑑𝑑) 2 × (0.25𝜋𝑑2 + 0.5𝜋𝑑ℎ + 𝑑ℎ) 0.5𝜋𝑑2 + 𝜋𝑑ℎ Here, S is surface, d is diameter and h is tablet thickness. Cancelling and rearrangement leads to the
following term:
2 × 𝑆(𝑑𝑑𝑑𝑑𝑑𝑑𝑑) 𝑆(𝑢𝑢𝑑𝑑𝑑𝑑𝑑𝑑𝑑) = 1 + 0.5𝜋𝑑 + 𝜋𝑑ℎ Here denominator (x + πh) is always greater than the numerator (2h), so overall result is less than 2. A lifelike value is due to diameter-height-ratio of conventional tablets. According to calculation, based on data of HD Medi, 2011, height represents in section 0,467 x diameter: 2 × 𝑆(𝑑𝑑𝑑𝑑𝑑𝑑𝑑) 𝑆(𝑢𝑢𝑑𝑑𝑑𝑑𝑑𝑑𝑑) = 1 + 0.5𝜋𝑑 + 0.467𝑑𝜋 ≈ 1 + 𝜋 ≈ 1.32 12 LIST, 1985 and SCHÖFFLING,2009 13 WELLENHOFER 2009 and 2010 14 SCHÖFFLING, 2009 and Identa 2009 Are divided pharmaceuticals in nursing homes replaceable? If there is no breaking notch, bisection leads to surface enhancement of 32 % at worst case scenario. However in case of an existing breaking notch, there is a surface enhancement of only 16 %, which is the half. Change in reaction progress It is well-established, that reaction velocity correlates with the surface of a reactant. More precisely it is proportional to the active surface, here named interface. Hence bisection increases degradation velocity of primary substance of maximum 32 % in case of monolithic pharmaceuticals without coating on equal terms. Pharmacokinetic contemplation Besides deviation influences pharmacokinetic of the unit dose. According to FICK´s law16 – 32 % surface enhancement increases also release velocity. But it is necessary to consider that patients should receive halved dose, which consists of the halved compact volume, too. The mentioned tablet properties give the fol owing dependence of diameters: 𝑑(0.5 𝑑𝑑𝑑𝑑) = Calculating ratio between 2 x surface(0.5 dose) and surface(dose) results in approximately 1.26. An unbrokenly tablet with the half dose obsesses a 26 % increased surface. That value is situated in the range of 16 % (breaking notch) and 32 % (without breaking notch). Surface enhancement is a countable, but not preventable influence of bisection. The legislator's implementation to prefer alternative pharmaceuticals instead of deviation means a huge change in release velocity. In addition long-term medication with commercially not available dosage are always a result of individual dosage finding of a physician.That´s why differences in liberation requires dosage adjustment and intensive monitoring. 15 EMIG & KLEMM, 2005 16 EMIG & KLEMM, 2005 17 QUINZLER & HAEFELI, 2006
Analytical Studies
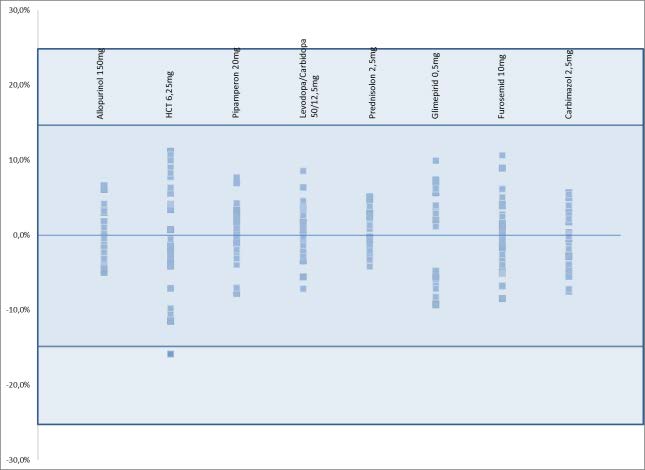
Dosage uniformity after deviation Each deviation provokes a certain amount of risk of irregular size distribution, which correlates with an oscillating dosage. Hence Ph. Eur. requires a special testing to ensure dosage uniformity. According to the current monograph of Pharmacopoeia, fractions of divisible tablets must fulfill the testing mass uniformity. Of course we investigated tablet fractions of our blistering routine in that content. Testing was performed by an analytical balance PCE AB 200 series with observational accuracy 0.1 mg. For each of the twenty analyzed dosages exists at least one ready-made pharmaceutical on the German market that fully satisfy pharmacopoeia´s requirements, se
Figure 1 – Testing of mass uniformity according to Ph. Eur. Deviation referring to fractions mean mass of the eight most
frequently divided dosages are il ustrated. Al generic medicaments (not displayed dosages, too) satisfy requires pharmacopeia´s
requirement.
Content uniformity Although uniformity of mass offers enough evidence for permissibility of tablet deviation, we want to establish proof of content uniformity according to Ph. Eur. 7. For the testing Allopurinol 150 mg, the most frequently blistered dosage, was chosen.
After unblistering ten tablets are picked up by chance and blistered via unit-dose procedure. A JV280 blistering machine was used. Afterwards the sachets were stored over 14 days according to pharmacopeia´s specification (maximum temperature 25 °C, maximum relative humidity 60 %) in
18 Testing 2.9.5, Ph. Eur., 2011
Are divided pharmaceuticals in nursing homes replaceable?
blistering boxes as secondary packaging for simulating stress maximum on the tablet fractions. Content determination was executed via HPLC based on specifications of Ph. Eur. 7.0.Whereas the following equipment was applied:
Merck-Hitachi L-6200 pump Rheodyne 7125 feeding valve Merck LiChrospher 100 RP-18e 250x4 mm chromatographic column Shimadzu SPD-6 detector, working at 230 nm wavelength
Evaluation offers a linear regression, characterized by R=0.9956 and a total y agreement with the requirements of Ph. Eur. 7.0. Determined mean content is 150.9 mg and al measured data match defined limits.
Mechanical stability studies The already mentioned contemplation and calculation are applicable only in case of dimensional stability of the fractions. Hence fractions must agree with Pharmacopeia´s friability (Ph. Eur. 7.0/ 2.9.7) and fracture strength (Ph. Eur. 7.0/ 2.9.8) specifications, too. Testing were performed by ERWEKA Tablet Hardness Tester TBH 125 series and Tablet Friability Tester TA100 series.
Alongside friability studies according to Pharmacopeia we were focused on tablet behavior towards mechanical stress. No cracked, damaged or broken tablet fraction was observed.
19 Content uniformity 2.9.6, Liquid chromatography 2.2.29 and Allopurinol monograph 7.0/ 0576
Figure 2 – Content determination of Al opurinol fractions with denomination 150 mg 14 days after blistering and storing in
sachets. Testing was carried out according to Pharmacopeia.
Are divided pharmaceuticals in nursing homes replaceable?
Tablet hardness testing confirms that illustration as well. Even though hardness varied substantially between 20 N and over 300 N, depending on direction of loading, it is possible to assure stability over storing period in the corresponding sachets. By the way there are no limits for measured data.
Figure 3 – Percentage friability of the eight frequently divided dosages are il ustrated. Al divided tablet fractions (not displayed
dosages, too) match the required percentage friability of 1 % (blue line).
Open-ended question Majority of the required dosages can be covered by fractioning, but there is a need of more data about a few, mainly coated, pharmaceuticals. Alongside the already mentioned analytical studies it is necessary to focus on dosage accuracy and durability, especially retention of liberation properties.
Accordant studies are in progress.
Are divided pharmaceuticals in nursing homes replaceable?
Each available study on durability of blistered pharmaceuticals was performed by storing tablets for some weeks in the cassettes before blistering. Therefore shelf life extension of at least one week for divided uncoated tablets is possible, if tablet fractioning takes place immediately after unblistering out of primary packaging.
If manufacturer defines a date of expiry it is permissible to reduce durability onto 75.8 % according to surface raise (1/ 1.32 %), see
If the following requirements are fulfilled, stability of the tablet fractions is assured:
Uncoated monolithic pharmaceuticals Stability evaluation and approval of the undivided tablet by the manufacturer Division immediately after withdrawal out of primary packaging and blistering immediately
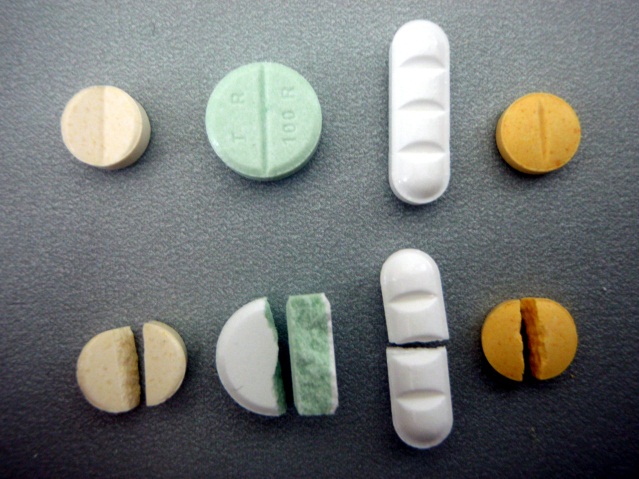
Figure 4 – Divisible pharmaceuticals. Outer face coloration gives no distinct evidence for a coating. As wel as dichroism, see
second one bottom left – matrix based tablet with modified liberation kinetics, is no criterion for exclusion.
With reference to an evaluation of former studies (concerning medicinal dosages, unreplaceable by total solid pharmaceuticals of 1,000 patients) three-fourths (71.7 %) of all dosages, accomplished by divided pharmaceuticals, can be supplied. Actual y there is at least one manufacturer, who will be able to fulfill
20 ALUID, 2011; HEXAL, 2012; Novartis, 2012; Ratiopharm, 2012
21 WELLENHOFER, 2010

Are divided pharmaceuticals in nursing homes replaceable?
the legal requirements. It is the blistering pharmacies´ job to proof mass uniformity of divided tablets and choose suitable generic pharmaceuticals if applicable.
Despite this approach, there is a dispute between the law (see Ordinance on the Operation of Pharmacies) and the implementation into daily routine by manufacturer, pharmacists and nursing staff. That topic should be discussed in the fol owing chapter.
Figure 5 - Examples not clearly divisible tablets and its fracture surface. Different coloration of outer face and core may be a
clear argument pro durability-modifying coating. Sometimes coating cannot identified before division (see second tablet
bottom left).
It is meaningful and necessary that pharmaceuticals offer an appropriate quality. Some paper remind a security hole in this mentioned quality of pharmaceutical supply. At first glance legislator bridges this security hole via reforming the Ordinance on the Operation of Pharmacies.
But here one respectively that essential point is disregarded: medication quality, which achieves the body. Finally service provider, from manufacturer to pharmacist and physician to nursing staff, must be reconciled with this aim. Only one definition of pharmaceutical quality can exist, which is a gladly phrased demand of ApBetrO, ABDA and representatives of public authorities. We agree with this important statement, but evaluation of pharmaceutical quality cannot be finished when leaving manufacturers platform or with the supply of N-packages. Rightly National Union of hospital- and nursing home supplying pharmacists (Bundesverband klinik- und heimversorgender Apotheker BVKA) point out that patient-individual dosage provision or blistering by pharmacies replace patient-individual dosage provision or blistering of non-pharmaceutical staff.Finally the legislator makes pivotal differences in quality of patient supplying. Thus supplying patients will be regulated as follows:
Centers of blistering as service provider of supplying pharmacies aren´t allowed to divide
pharmaceuticals without exception
Pharmacies, providing or blistering themselves, are allowed to divide pharmaceuticals only in
exceptional cases
Nursing staff in the context of patient-individual dosage providing are allowed to divide
pharmaceuticals in each case
It should be difficult to found this distinction by law.Even justification considering quality of pharmaceutical supply should be impossible.
Additionally implementation of legal quality demand must be ensured until application. But why applies that demand only in case of patient-individual dosage provision by a pharmacy? Nursing homes as wel provide patient-individual dosage mostly at the beginning of a week, divided pharmaceuticals included. But durability is neither depending on the place of division nor on the dividers´ occupational title. Besides nursing staff is not able to evaluate durability better than a pharmacist for sure, which shows the discrepancy of the legislators´ regulations.
But there are further questions: Does pharmaceutical´s authorization combined with the specified divisibility implement durability for the divided dosage form, too? The answer is no. On the one hand this would be meaningful and would correspond to the basic requirements on the other hand. Because patient-individual dosage providing in nursing homes is common practice upon approval by the authorities. Appointing durability to one week would be the consequence, otherwise approximately 2.25 billion of supplied patients with and without hospitalization would receive potentially expired medicine. This situation isn´t detectable in the circles of experts. However not later than ApBetrO coming into force authorities are requested to harmonize durability statements in the context of pharmaceutical authorization with the real situation. This is a demand of Pharmacists' associations on manufacturers.
22 RIESENBERGER, 2007; SCHMIDT et al., 2008
23 Position paper BVKA, 2012
24 See §34 2.6, CYRAN/ ROTTA, 09/2012
Are divided pharmaceuticals in nursing homes replaceable?
Hydrochlorothiazide 6.25
Benserazide Quetiapine
Triamterene comp.
Hydrochlorothiazide 6.25
Are divided pharmaceuticals in nursing homes replaceable?
Table 1 - Only by bisection reachable dosages of oral long-term medication and its potential for division prohibiting. Data pool:
1,531 patients supplied by automatic patient-individual blistering (2013).
Even if durability appointment to minimum one week isn´t in keeping with the law, there is occurring the question of how to look on tablet division in hospitals and nursing homes.
Is patients´ treatment de jure carried out with expired pharmaceutical? Is the application of divided tablets in advance a kind of off-label-use? Does liability in this
context move to the physician?
How to proceed with dosages, only reachable by division of a tablet in the future?
There are already existing recommendations to avoid bisection. VERRUE (2011) suggests use of Liquida. But only about 32 % of all primary substances are available in a liquid type, see In addition there are conflicts with current rules of prescribing: Above all the new regulations regarding division of pharmaceuticals get physicians under pressure, because there are GKV-made restrictions in prescription of liquid pharmaceutical, as the only meaningful alternative for adults.
But there is another suggestion by the BPAV (2012): Tablets in order of division should be provide as intact pharmaceuticals and divide just before application. However the dangers of overdosage aren´t take into consideration. Besides BPAV don´t focus on the financial aspects of a 50-per-cent-disposal. Shift of responsibility for an inevitable division, in the patients´ interest, to the nursing staff raises the already mentioned liability problems.
That´s why targeted studies of selected, otherwise not replaceable, divided pharmaceuticals are indispensable, if manufacturer don´t close known gaps of long-term medicationsupply in the near future. To do justice to the multiplicity of division, developing a hazard analysis is necessary. Besides the results can be used for targeted studies.
However we have to point out, that the new rules are a step in the right direction. The fad of dividing pharmaceuticals without apparent reasonlead back to a meaningful rate. With reference to the wording of the law it wouldn´t be al owed to deliver about 12.5 % of the dosages, which should make persons in charge thought-provoking as well as the striking unequal evaluation of different supply types.
25 See the contracts between general health insurance companies (GKV) and association of GKV physicians
26 Appendix II , guideline of pharmaceuticals
27 WELLENHOFER, 2010
28 QUINZLER et al., 2006; WELLENHOFER, 2009; VERRUE, 2011
Are divided pharmaceuticals in nursing homes replaceable?
By so fare partially closed statements public authorities try to avoid this dilemma in a feasible way. So Federal Ministry of Health tells medical association in April 2013, to divide tablets only in case of clinical need and permissibility in a pharmaceutical way of view. The Regional Chamber of Pharmacists for Bavaria deliver an opinion, too (March 2013). Tablets should be divided correctly, so that pharmaceuticals don´t lose quality, which would lead to an improper supply or harm of patients. Hopefully implementation of ApBetrO will exclusively occur in the patients´ spirit.
Are divided pharmaceuticals in nursing homes replaceable?
Permissibility rules of division of pharmaceuticals in the context of patient-individual dosage provision and blistering closes at least unsolved problems partially. But many questions are already unanswered or newly arising. Manufacturers´ durability data of uncoated pharmaceuticals furnish evidence, that specifications of undivided tablets are transmittable to the divided form in a modified way. Testing of the mostly used dosages confirm this thesis. Actually overall manufacturers cover about 72 % of long-term medication dosages, unable to supply by unbroken pharmaceuticals and with curtailment further 16 % by Liquida.
Are divided pharmaceuticals in nursing homes replaceable?
ABDA-DB. ABDATA, 2012 Ärztezeitung: Bald Schluss mit der Verordnung geteilter Tabletten? Ausgabe vom 16.04.2012 Aliud: www.aliud.de; Portfolio (nur für Fachkreise zugänglich); Mai 2012 Aliud: Risikoanalyse finale Version. Persönliche Korrespondenz; 2011 BLAK: Kammerrundschreiben 03/2013; S. 13 BMG: Nachträgliches Teilen von Tabletten. Brief an die Bundesärzteammerund die Arbeitsgemeinschaft der deutschen Ärztekammern. April 2013 BMG: Verordnung über die Anwendung der Guten Herstel ungspraxis bei der Herstel ung von Arzneimitteln und Wirkstoffen und über die Anwendung der Guten fachlichen Praxis bei der Herstellung von Produkten menschlicher Herkunft (Arzneimittel- und Wirkstoffherstel ungsverordnung – AMWHV). 22.12.2011 BMG: Vierte Verordnung zur Änderung der Apothekenbetriebsordnung. 11.06.2012 BPAV: Pressemitteilung vom 04. April 2012. www.blisterverband.de BVKA: Stel ungnahme des Bundesverbandes klinik- und heimversorgender Apotheker e.V. (BVKA) vom 06. Januar 2012 zum Referentenentwurf für ein Zweites Gesetz zur Änderung arzneimittelrechtlicher und anderer Vorschriften. www.bvka.de; 2012 Cyran/Rotta: Apothekenbetriebsordnung Kommentar. September 2012 Duden: Das Fremdwörterbuch. Dudenverlag, 2001; ISBN 3-411-04057-2 Eming Gerhard, Klemm Elias: Technische Chemie: Einführung in die Chemische Reaktionstechnik. Springer Verlag; 2005 ISBN 3-540-23452-7 EUAB: Europäisches Arzneibuch 7. Ausgabe Grundwerk 2011; Deutscher Apothekerverlag; Stuttgart & Govi-Verlag; Eschborn HD Medi: canisterparts. Excel-Tabelle / interne Daten. September 2011 Hexal: www.hexal.de; Portfolio (nur für Fachkreise zugänglich); Mai 2012 Identa Gelbe Liste 2009. Medizinische Medien Informations GmbH; Neu-Isenburg; 2009 ISSN 1616-198X List Paul Heinz: Arzneiformenlehre. Wissenschaftliche Verlagsgesellschaft mbH Stuttgart; 1985 ISBN 3-8047-0813-7 Novartis: Neue Herausforderungen an die Produktstabilität. Zur Veröffentlichung geplant. April 2012; persönliche Korrespondenz Quinzler R, Gasse C, Schneider A, Kaufmann-Kol e P, Szecsenyi J, Haefeli WE: The frequency of inappropoate tablet splitting in primary care. Eur J Clin Pharmacol [2006) 62: 1065-1073 Quinzler R, Haefeli WE: Tabletten teilen. Therapeutische Umschau (2006) Band 63 Heft 6: 441-447 Ratiopharm: HB Pharmaconsult:Betrachtungen zur Stabilität von sekundärverblisterten Produkten. 11/2009 Riesenberger Michael: Patientenindividuelle Zweitverblisterung. DAZ 48/2007; S 44-54
Are divided pharmaceuticals in nursing homes replaceable?
Schmidt M, Terhechte A, Schieweck A, Zeitler K: Maschinelles patientenindividuelles Verblistern von Arzneimitteln. Modul Technische Aspekte. Apothekerkammer Niedersachsen 2008 Schöffling Ursula, Grabs Silvia: Arzneiformenlehre. Deutscher Apotheker Verlag; 2009; ISBN 978-3-7692-4093-1 Verrue Charlotte, Mehuys Els, Boussery Koen, Remon Jean Paul, Petrovic Mirko: Tablet-splitting: a common yet not so innocent practice. Adv. Nurs. 67; 2011; S 26-32 Wellenhofer Thomas: Tablettenteilungen. Zur Wirtschaftlichkeit in Heim und Pflegeambulanz. Pharm Ztg 13/2009; S 64-67 Wellenhofer Thomas: Geteilte Tabletten in Heimen und Pflegeambulanz. DAZ 3/2010; S 66-69
Source: http://www.ikatse.de/wa_files/is_it_necessary_to_proof_drugs_durability_in_case_of_deviation.pdf
fooddiagnostics.dk
Product Catalogue Food & Feed Analysis R-Biopharm – for the safety of your analysis. R-Biopharm AG – Product Catalogue 2016 • ELISA, Lateral Flow and real-time PCR R-Biopharm AG – Product Catalogue 2016 Overview of test systems ELISA – RIDASCREEN®• Quantitative results• Applications for many matrices• Analysis by RIDA®SOFT Win• Can be automated
katelarisurology.com.au
PULL OUT & KEEP UPDATE LOWER URINARY TRACT SYMPTOMS IN MEN This Update discusses lower urinary tract symptoms in men, outlines the appropriate investigations and describes the management options. DR PHILLIP KATELARIS FRACS (UROL)Consultant Urological Surgeon Confl icts: nothing to declare DR CLAIRE BERMAN MB.BCH