Wus and stm in shoot meristem regulation
Development 129, 3195-3206 (2002) Printed in Great Britain The Company of Biologists Limited 2002DEV0437 The WUSCHEL and SHOOTMERISTEMLESS genes fulfil complementary rolesin Arabidopsis shoot meristem regulation
Michael Lenhard1, Gerd Jürgens2 and Thomas Laux1,*
1Institut für Biologie III, Universität Freiburg, Schänzlestrasse 1, D-79104 Freiburg, Germany2Universität Tübingen, ZMBP – Entwicklungsgenetik, Auf der Morgenstelle 1, D-72076 Tübingen, Germany*Author for correspondence (e-mail: [email protected]) Accepted 9 April 2002 Continuous organ formation from the shoot apical
and STM activities induce the expression of different
meristem requires the integration of two functions: a set of
downstream target genes. Finally, the pathways regulated
undifferentiated, pluripotent stem cells is maintained at the
by WUS and STM appear to converge in the suppression of
very tip of the meristem, while their daughter cells in the
differentiation, since coexpression of both genes produced
periphery initiate organ primordia. The homeobox genes
a synergistic effect, and increased WUS activity could
partly compensate for loss of STM function. These results
encode two major regulators of meristem formation and
suggest that WUS and STM share labour in the shoot apical
maintenance in Arabidopsis, yet their interaction in
meristem: WUS specifies a subset of cells in the centre
meristem regulation is presently unclear. Here, we have
as stem cells, while STM is required to suppress
addressed this question using loss- and gain-of-function
differentiation throughout the meristem dome, thus
approaches. We show that stem cell specification by WUS
allowing stem cell daughters to be amplified before they are
does not require STM activity. Conversely, STM suppresses
incorporated into organs.
differentiation independently of WUS and is required and
sufficient to promote cell division. Consistent with their
Key words: Arabidopsis, SHOOTMERISTEMLESS, WUSCHEL, independent and distinct phenotypic effects, ectopic WUS
stem cells, shoot meristem 16-cell stage embryo and later becomes restricted to a smallcentral cell group underneath the presumed stem cells in the Postembryonic development of higher plants is characterized outermost three cell layers. Thus, WUS expression appears to by the continuous formation of organs from the shoot apical define an organizing centre whose activity establishes an apical meristem (SAM) (Steeves and Sussex, 1989). The SAM serves group of long-term stem cells. two main functions: in the central zone, a population of WUS expression is under negative control by the CLAVATA undifferentiated, pluripotent stem cells is maintained, and in genes (CLV1, CLV2 and CLV3), which encode components of the peripheral zone, lateral organ primordia are initiated. While a presumed receptor-kinase signal transduction pathway (Clark all cells of the meristem dome remain undifferentiated until et al., 1997; Jeong et al., 1999; Fletcher et al., 1999). In clv they are incorporated into organ primordia, only a specialized mutants, the SAM enlarges progressively by the accumulation subset functions as long-term stem cells from which all cells of stem cells (Clark et al., 1993; Clark et al., 1995; Fletcher et of the shoot and its lateral organs are ultimately derived (Satina al., 1999), and this enlargement appears to be a consequence et al., 1940; Stewart and Dermen, 1970). These stem cells are of ectopic WUS expression in more apical and lateral cells in located in three cell tiers at the very apex and coincide with clv mutant SAMs (Schoof et al., 2000). This has led to a model the domain where the CLAVATA3 (CLV3) gene is expressed in which stem cell maintenance is regulated by a negative (Fletcher et al., 1999).
feedback loop mediated by the WUS and CLV3 genes, with the Genetic analysis in Arabidopsis has identified two major organizing centre signalling to the apical neighbours to specify regulators of SAM formation and maintenance, the homeobox them as stem cells, which in turn signal back to restrict the size genes WUSCHEL (WUS) and SHOOTMERISTEMLESS of the organizing centre (Brand et al., 2000; Schoof et al., (STM). In wus mutants the apical stem cells are unable to self- maintain (Laux et al., 1996; Mayer et al., 1998), whereas Loss-of-function mutations in the SHOOTMERISTEMLESS ectopic WUS expression can abolish organ formation at the (STM) gene, which encodes a homeodomain protein of the SAM and induce expression of the putative stem cell marker KNOTTED class (Long et al., 1996) also result in a lack of a CLV3 (Schoof et al., 2000). During embryogenesis, WUS self-maintaining meristem. Instead of forming a SAM, the cells mRNA can first be detected in the four inner apical cells of the in the apex of stm mutant embryos appear to differentiate 3196 M. Lenhard, G. Jürgens and T. Laux (Barton and Poethig, 1993; Endrizzi et al., 1996). In addition, previously (Schoof et al., 2000). In all cases, samples to be compared stm mutant seedlings exhibit fusion of the cotyledon petioles, where stained for the same duration.
suggesting that STM fulfils two functions: it inhibits differentiation of the cells in the embryo apex and preventsoutgrowth of the cells separating the cotyledon primordia in Plants were genotyped for the wus-1 allele by dCAPS (Neff et al.,1998) as described by Groß-Hardt et al. (Groß-Hardt et al., 2002).
the periphery. Repression of differentiation by STM in theSAM primordium appears to occur mainly via repression of Construction of transgenes and plant transformation
the MYB-related gene ASYMMETRIC LEAVES1 (AS1), since For all misexpression experiments we used the pOpL two-component loss of AS1 function in an stm mutant background rescues system, where a promoter of interest controls the expression of a SAM formation (Byrne et al., 2000). STM mRNA is expressed synthetic transcription factor, LhG4 (Moore et al., 1998). The gene to in the shoot meristem primordium from the globular embryo be expressed is controlled by a synthetic promoter, pOp, which is stage on, and postembryonically expression is found specifically activated by LhG4. For the sake of simplicity, we will throughout the SAM, but is excluded from incipient organ refer to plants, for example, of the genotype ANT::LhG4; pOp::STM primordia (Long et al., 1996). as ANT::STM.
Whether and how the regulatory pathways defined by WUS Generation of the pOp::WUS-pOp::GUS (MT72) transgenic line, as well as of ANT::LhG4 and CLV1::LhG4 lines was described before and STM interact in SAM formation and maintenance is (Schoof et al., 2000). presently unclear. However, several lines of evidence have been For the pOp::STM construct, the STM coding region was isolated taken to suggest that WUS is a downstream target of STM in from pCGN1547:35S::STM (kindly provided by R. Williams) by functional SAMs: wus mutations exacerbate the phenotype of digestion with BamHI and subcloned into pU-BOP (kindly provided weak stm loss-of-function alleles, while strong stm mutations by I. Moore) which had been digested with BamHI. The resulting are epistatic to wus (Endrizzi et al., 1996); STM exhibits pOp::STM fragment was excised from pU-BOP:STM by partial dosage-sensitive interactions with the CLV genes (Clark et al., digestion with SacI and HindIII and subcloned into pBarM, a 1996), suggesting that STM and CLV may act antagonistically derivative of pGPTV-BAR (Becker et al., 1992), linearized with SacI on common downstream targets, one of which could be WUS; and HindIII to yield plasmid MT153. For the pOp::STM-pOp::GUS although WUS expression is initiated correctly in stm mutants, construct, a pOp::GUS fragment was isolated from plasmid MT162by digestion with EcoRI and inserted into plasmid MT153 to yield it is not maintained in later embryo stages (Mayer et al., 1998).
However, WUS expression is initiated earlier in embryogenesis For the 35S::WUS-GR construct, the WUS open reading frame was than STM expression (Mayer et al., 1998; Long and Barton, amplified using primers WUS5BAM (5′-AGT CGG GAT CCA CAC 1998), arguing that at least in embryonic SAM formation there ACA TGG-3′) and WUS3BAM+2 (5′-GAG CGG ATC CAG ACG is no linear pathway with WUS downstream of STM.
TAG CTC AAG AG-3′), digested with BamHI and subcloned into To understand how the functions of WUS and STM are the BamHI site of pRS020 (kindly provided by R. Sablowski) integrated in SAM regulation, we have analyzed their which contains the coding sequence of the C terminus of the rat interactions, using a combination of loss- and gain-of-function glucocorticoid receptor (GR), producing an N-terminal fusion of WUS to GR (MT141). The WUS fragment was sequenced to excludeamplification errors. The resulting WUS-GR fusion gene was insertedas an XbaI/SmaI-fragment into pBar35S (kindly provided by G.
Cardon) to yield MT142.
MATERIALS AND METHODS
Generation of the WUS::NLSGUS and CLV3::NLSGUS constructs have been described previously (Groß-Hardt et al., 2002).
Mutant lines, growth conditions and dexamethasone
All constructs were introduced into Agrobacterium strain GV3101 (pMP90) (Koncz and Schell, 1986) by electroporation. Arabidopsis The wild type used in all experiments was the Landsberg erecta (Ler) wild-type plants were transformed by floral-dip (Clough and Bent, ecotype. The wus-1 mutant has been described previously (Laux et al., 1996; Mayer et al., 1998), as well as the stm5 mutant (Endrizzi et KNAT1::GUS transgenic plants were kindly provided by S. Hake; al., 1996). stm-5 carries a G to A transition of the first nucleotide of the KNAT2::GUS line was obtained from J. Dockx and J. Traas, and the third intron, which changes the conserved GA dinucleotide of the the CycB1;1::CDBGUS line was a gift from J. Celenza. In this exon-intron boundary to AA and is predicted to prevent the intron construct, the cyclin-destruction-box (CDB) of CycB1;1 is fused in from being spliced out. This would result in a translational stop after frame to GUS, causing the protein to be degraded at the end of mitosis, the addition of ten unrelated amino acids, causing a loss of the allowing visualization of cell-cycle progression by staining for GUS C-terminal half of the homeodomain (A. Haecker and T. L., unpublished). Plant growth conditions were as described previously(Laux et al., 1996). For dexamethasone induction, plants were sprayed In situ hybridization
with a solution of 5 µM dexamethasone (Sigma Aldrich; St. Louis, In situ hybridization for WUS and CLV3 was performed as described USA)/0.015% Silwet L-77 (OSi Specialties; Meyrin, CH) in tap water.
by Mayer et al. (Mayer et al., 1998) and Schoof et al. (Schoof et al., For the mock treatment, 0.025% ethanol/0.015% Silwet L-77 in tap water was used, since the dexamethasone stock solution was 20 mM For the KNAT1 riboprobe, the KNAT1 cDNA was amplified from in 100% ethanol. Seedlings were harvested 2 days after induction.
reverse transcribed poly(A)+ RNA of Landsberg erecta seedlingsusing primers KNAT1-FOR (5′-TCT CTC GAG TCT TTA CTC ATC Histology, scanning electron microscopy and GUS
TGG G-3′) and KNAT1-REV (5′-AAA GGA TCC GTT GTA ACA AGA AAG C-3′). After digestion with XhoI and BamHI, the cDNA Preparation of histological sections from LR-White embedded was inserted into pBluescript II KS–. The C-terminal part, containing material, DAPI staining of seedlings and scanning electron the homeobox, was removed by digestion with XbaI and religation to microscopy were done as described previously (Laux et al., 1996; yield ML343. For the antisense probe, ML343 was linearized with Schoof et al., 2000). GUS staining was performed as described XhoI and transcribed with T7 RNA polymerase (Promega; Madison,
WUS and STM in shoot meristem regulation 3197
USA) using a digoxigenin-labelling kit (Roche Diagnostics;
We showed that no significant cross hybridization could occur
Mannheim, Germany); for the sense probe, ML343 was linearized
between the KNAT2 antisense riboprobe and KNAT1 mRNA by a filter
with XbaI and transcribed with T3 RNA polymerase (Promega;
hybridization experiment that mimicked the conditions of in situ
Madison, USA).
hybridization (data not shown).
For the KNAT2 antisense riboprobe, plasmid pCKI-30 (kindly
provided by J. Traas) which contains the full-length KNAT2 cDNAwas linearized with XhoI and transcribed with T7 RNA polymerase;
for the sense probe, pCKI-30 was linearized with HindIII andtranscribed using SP6 RNA polymerase (Promega; Madison, USA).
Ectopic expression of STM in leaf primordia
For all comparisons of wild-type and mutant or transgenic
suppresses cell differentiation
seedlings, sections from plants of the two genotypes under study were
Based on its expression pattern and loss-of-function
hybridized on the same slides, and only those slides were included in
phenotype, STM
appears to maintain cells in an
the analysis that showed clear expression in the wild-type samples.
Where expression is reported, this was observed in several serial
undifferentiated state, before they are incorporated into leaf
sections. The numbers given for CLV1::WUS-expressing stm5
primordia. To test whether STM was sufficient to suppress
mutants refer only to those seedlings that contained an adventitious
differentiation, we expressed STM ectopically in leaf
primordia, using the pOpL two-component system (Moore etal., 1998; see Materials and Methods). The functionality of theSTM transgene was confirmed by complementation of themeristem defect in stm5 homozygous mutants (Fig. 1A-D).
We expressed STM under the control of the
AINTEGUMENTA (ANT) promoter, which shows acomplementary expression pattern to that of STM, i.e. it isactive in primordia of cotyledons and leaves (Elliott et al.,
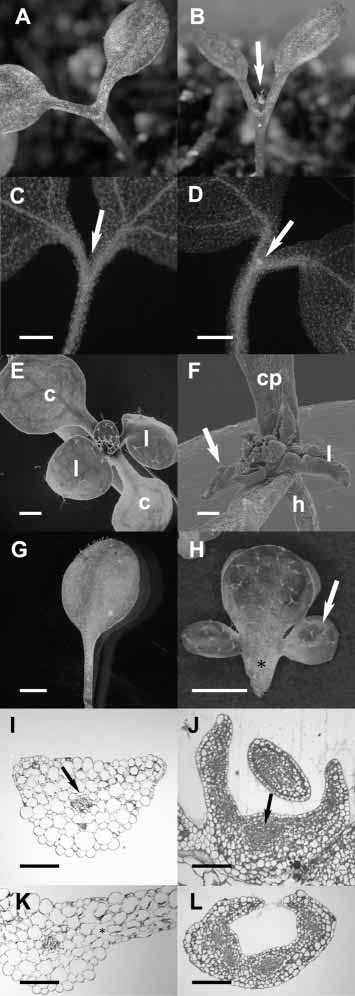
Fig. 1. Ectopic STM expression suppresses cell differentiation.
(A) Light micrograph of a non-transgenic stm5 mutant seedling 8
days after germination. Cotyledon petioles are fused and no leaves
have been formed. (B) Light micrograph of an stm5 mutant seedling
expressing CLV1::STM 8 days after germination. The first pair of
leaves formed by the SAM is visible (arrow). The bases of the
cotyledon petioles are fused as in the seedling shown in A. We used
the CLV1 promoter, which is active in the centre of the embryonic
shoot meristem primordium from heart-stage onward, and whose
initial activation does not require STM function (Long and Barton,
1998), since no STM promoter has been described that mimics the
endogenous mRNA expression pattern. (C,D) Micrographs of DAPI-
stained seedlings. (C) stm5 mutant seedling 5 days after germination.
No meristematic cells are visible inside the fused cotyledon petioles
(arrow). (D) CLV1::STM-expressing stm5 mutant seedling 5 days
after germination. A meristematic region is evident from the bright
signal from cytoplasmically dense cells inside the fused petioles
(arrow). (E,F) Scanning electron micrographs. (E) Wild-type seedling
10 days after germination. c, cotyledon; l, leaf. (F) ANT::STM-
expressing seedling with a strong phenotype 21 days after
germination. The petioles of the cotyledons (cp) are broader than in
wild type (compare with E). Leaves (l, arrow) are not expanded and
are rolled up at their margins. h, hypocotyl. (G) Light micrograph of
a mature second rosette leaf of a wild-type plant. (H) Light
micrograph of a mature second rosette leaf of an ANT::STM-
expressing plant with a weak phenotype. The petiole (asterisk) is
broader than wild type and lateral outgrowths have developed into
leaf-like structures (arrow). (I-L) Cross-sections of plastic-embedded
leaf material from seedling 12 days after germination, stained with
Toluidine Blue. (I) Petiole of the first rosette leaf of a wild-type plant.
A vascular bundle (arrow) with differentiated cells lacking cytoplasm
is surrounded by large, vacuolated cells. (J) Basal part of the first
rosette leaf of an ANT::STM-expressing seedling. The cells in place
of the vascular strand (arrow) are cytoplasmically dense and the cells
throughout the petiole are less expanded than in G. (K) The lamina of
the first rosette leaf of a wild-type plant. Note the high degree of
vacuolation and the large intercellular spaces (asterisk). (L) The
lamina of the first rosette leaf of an ANT::STM-expressing seedling.
Cells throughout the leaf are smaller than in I and contain more
cytoplasm, indicating that differentiation is suppressed. Scale bars are
500 µm in C-H, 100 µm in I-L.
3198 M. Lenhard, G. Jürgens and T. Laux
1996; Klucher et al., 1996). Staining for the activity of a
2C), consistent with our observation that these arose after the
linked ANT::GUS reporter gene confirmed expression of
main leaf had already reached a certain size (data not shown).
the transgenes in cotyledons and leaf primordia (Fig. 2D).
This result suggests that ectopic STM expression in cells of leaf
ANT::STM-expressing plants showed cotyledon and leaf
primordia promotes their proliferation.
phenotypes of varying severity, depending on the individual
Since the leaf phenotype of ANT::STM-expressing plants
STM target line used. The petioles of the cotyledons and of
was similar to the effects observed when either KNAT1 or
leaves were up to approximately threefold wider than in non-
KNAT2, two homeobox genes with potential regulatory
transgenic plants (Fig. 1E-H). Leaves were smaller than in wild
functions in the shoot meristem, were overexpressed (Lincoln
type, and in the most extreme cases, were reduced to small
et al., 1994; Dockx et al., 1995; Chuck et al., 1996; Pautot et
finger-like structures (Fig. 1F, arrow). Their dorsoventrality
al., 2001), we addressed whether KNAT1 or KNAT2 was acting
was maintained, however, as judged from the development of
in one regulatory pathway with STM. Staining for a
trichomes only on the adaxial side of early vegetative leaves
KNAT1::GUS reporter revealed ectopic expression in the
and their anisotropic growth, causing the leaves to bend over
vasculature of the cotyledons and in strongly affected leaves of
the SAM as they do in wild type. Furthermore, leaves of the
ANT::STM-expressing seedlings (Fig. 2E,F), suggesting that
transgenic plants developed lateral outgrowths from the leaf
ectopic KNAT1 expression can be activated by ectopic STM
blade or petiole which was never observed in wild type (Fig.
activity. Similarly, the KNAT2::GUS reporter showed ectopic
staining in the vasculature of the cotyledons and in leaves of
Histological analysis showed that differentiation of leaf cells
ANT::STM-expressing seedlings (Fig. 2G,H).
was suppressed in ANT::STM-expressing leaves compared to
In contrast to KNAT1 and KNAT2, the stem cell marker CLV3
wild type. In the most severe cases we did not observe a
was not expressed ectopically in ANT::STM-expressing
vascular bundle in the finger-like structures at a time when
seedlings: using in situ hybridization CLV3 RNA was only
wild-type petioles contained a well differentiated vascular
detected in the apical stem cells of the shoot meristem, which
strand (Fig. 1I,J). In addition, the cells throughout the leaf were
was indistinguishable from wild type (Fig. 2I,J).
small and cytoplasmically dense, resembling meristematic
Thus, ectopic expression of STM in leaf primordia induces
cells in contrast to the large, vacuolated differentiated cells of
expression of two meristem genes and promotes cell
wild-type leaves (Fig. 1K,L).
proliferation, yet STM is not able to induce ectopic stem cell
Thus, STM is able to suppress cell differentiation in
identity, based on expression of the presumed stem cell marker
developing leaves and instead maintains the potential to form
additional lateral outgrowths. These results support thereported phenotype of 35S::STM-expressing plants which have
WUS induces ectopic stem cell identity, but not the
a stunted appearance with a disorganized shoot and leaf-like
expression of KNAT genes
bulges that do not develop into mature leaves (Williams, 1998).
To molecularly delimit the functions of STM and WUS, we
However, the effects of ectopic STM expression in leaf
aimed to test whether expression of the above marker genes
primordia are relatively subtle compared to those of
could be induced by ectopic WUS activity in leaves,
ANT::WUS expression, which entirely abolishes organ
complementary to the analysis for STM. Since constitutive
formation (Schoof et al., 2000).
ANT::WUS expression completely suppresses leaf formation(Schoof et al., 2000), we used an inducible construct to
STM induces the expression of KNAT genes and
produce leaves with ectopic WUS activity: we expressed a
CycB1;1, but not stem cell identity
posttranslationally inducible form of WUS fused to the C
In order to molecularly characterize the effects of ectopic STM
terminus of the rat glucocorticoid receptor (GR) (see Sablowski
activity, we analyzed the expression of several candidate
and Meyerowitz, 1998) from the constitutive Cauliflower
downstream genes in ANT::STM-expressing plants.
Mosaic Virus 35S promoter. Nuclear translocation of this fusion
The formation of lateral outgrowths by ANT::STM-
protein, and thus its potential to activate transcription, can be
expressing leaves suggested that STM was able to promote cell
induced by addition of a GR-ligand such as dexamethasone.
proliferation when expressed in leaves. To test this, we
When germinated on dexamethasone-containing medium,
examined the expression of the mitotic cyclin CycB1;1 using
35S::WUS-GR
seedlings are indistinguishable from 35S::WUS
a promoter-GUS fusion. CycB1;1 is expressed shortly before
seedlings with suppressed differentiation, whereas in the
and during mitosis and overexpression analysis suggests it may
absence of dexamethasone the transgene has no effect on plant
be a limiting factor for cell division, making it a suitable
development as has dexamethasone treatment of 35S::GR-
marker for mitosis and cell proliferation (Doerner et al., 1996;
expressing seedlings, indicating that the fusion protein behaves
Mironov et al., 1999).
as predicted and that the effects observed are due to ectopic
In 10-day old wild-type plants carrying a
WUS activity (Fig. 3A; data not shown). We introduced GUS
CycB1;1::CDBGUS
reporter gene, GUS staining was
reporter genes for CLV3, KNAT1, KNAT2 and CycB1;1 into
restricted to the shoot meristem and young leaf primordia, but
35S::WUS-GR seedlings and analyzed GUS activity in 14-day
was absent from the expanding first pair of leaves (Fig. 2A).
old F1 seedlings that had been treated for 2 days with
In ANT::STM; CycB1;1::CDBGUS seedlings the first pair of
dexamethasone or with a control solution.
leaves became visible at the same time as in wild type, yet still
Dexamethasone induction of 35S::WUS-GR seedlings
showed GUS staining at 10 days after germination, in addition
resulted in strong ectopic activation of the CLV3::NLSGUS
to staining in the shoot meristem with younger leaf primordia
reporter gene in cotyledons, leaves and hypocotyl, mainly
(Fig. 2B). In older ANT::STM-expressing leaves, ectopic GUS
associated with the vasculature (Fig. 3D), whereas uninduced
staining was most pronounced in the lateral outgrowths (Fig.
siblings showed GUS staining exclusively in the apical stem
WUS and STM in shoot meristem regulation 3199
Fig. 2. Marker gene expression in ANT::STM plants. (A-H) Light
micrographs of GUS-stained, cleared seedlings.
(A) CycB1;1::CDBGUS expression in wild type. Staining is
restricted to the SAM region and young leaves (arrowhead), but is
absent from the expanded first pair of rosette leaves (arrow).
(B) CycB1;1::CDBGUS; ANT::STM-expressing seedling of the same
age as the one in A. Staining is seen throughout the first pair of
rosette leaves (arrow). (C) CycB1;1::CDBGUS; ANT::STM seedling
with intermediate phenotype. Ectopic GUS staining is observed in
the lateral outgrowths of the leaves (arrows). (D) ANT::STM;
ANT::GUS-expressing seedling. The transgenes are strongly
expressed in the vasculature of the cotyledons (c), leaf primordia
(arrowhead) and in older leaves with stronger staining at the tips
(arrow), as well as in their lateral outgrowths (not visible).
(E) KNAT1::GUS expression in wild type. Staining is restricted to
the SAM region and hypocotyl, yet is absent from leaves.
(F) KNAT1::GUS; ANT::STM-expressing seedling. Ectopic GUS
staining is seen in the vasculature of the cotyledons (c) and in
strongly affected leaves (arrow). (G) KNAT2::GUS expression in
wild type. Staining is restricted to the SAM region and is absent from
cotyledons (c) and leaves (arrow). (H) KNAT2::GUS; ANT::STM-
expressing seedling. Ectopic GUS staining is observed in the
vasculature of the cotyledons (c) and in leaves (arrow). (I,J) In situ
hybridization with a CLV3 antisense riboprobe. In both wild-type (I)
and ANT::STM-expressing (J) seedlings, CLV3 mRNA is exclusively
detected in the stem cells in the three outermost layers of the SAM.
Scale bars are 1 mm in A-H, 100 µm in I,J.
In summary, WUS is sufficient to induce ectopic stem cell
identity – as judged by CLV3 expression – and occasionalectopic cell divisions, but is not able to ectopically activateexpression of KNAT1 or KNAT2. Taken together, these resultssuggest that ectopic expression of STM or WUS in leafprimordia activates distinct sets of downstream target genes.
Ectopic STM and WUS functions act independently
of each other
To study how the activities of WUS and STM are
interconnected, we analyzed whether the activity of one gene
is required for the effects of ectopic expression of the other
gene in leaf primordia.
To analyze whether STM might be a downstream target of
WUS, we tested whether ectopic WUS expression could stillrepress organ formation in an stm5 mutant background. WhileANT::WUS expression in a wild-type background produced
cells of the SAM (Fig. 3B,C). Thus, WUS appears to be
an enlarged SAM in place of leaves immediately after
sufficient to induce aspects of stem cell identity de novo in
germination, no effect of the transgene was observed in stm5
differentiated tissue. Preferential induction close to the
mutant seedlings up to 7 days after germination. However,
vasculature could either be due to predominant expression of
thereafter ANT::WUS-expressing stm5 mutant seedlings
the 35S promoter there (e.g. Chuck et al., 1996) or to a higher
formed a mass of small meristematic cells inside the fused
sensitivity of cells near the vasculature to WUS activity.
cotyledon petioles that was indistinguishable from that
By contrast, expression of neither the KNAT1::GUS nor the
observed in ANT::WUS-expressing wild-type seedlings (Fig.
KNAT2::GUS reporter genes could be induced ectopically by
4A,B,D,E). The relatively late effect in stm5 mutants
35S::WUS-GR (Fig. 3E-H), indicating that WUS-GR is not
compared to wild type appears to be due to the fact that the
able to activate expression from the KNAT1 and KNAT2
transgene is not expressed in stm5 mutants up to 7 days after
germination, as judged from staining for the activity of a
In dexamethasone-induced 35S::WUS-GR seedlings
linked ANT::GUS reporter gene (data not shown), and
carrying the CycB1;1::CDBGUS reporter, we occasionally
expression only becomes detectable thereafter (Fig. 4C). By
detected ectopic staining in the first pair of leaves (5 out of 15
contrast, non-transgenic stm5 seedlings never produced a
seedlings analyzed) which was never detected in uninduced
similar enlarged SAM, but formed adventitious leaves
seedlings of the same genotype (Fig. 3I; n=15). The ectopically
between the fused cotyledon petioles (Fig. 4F) (Endrizzi et al.,
stained cells were always associated with the vasculature.
3200 M. Lenhard, G. Jürgens and T. Laux
Fig. 3. Marker gene expression in 35S::WUS-GR-expressing plants.
(A) 35S::WUS-GR-expressing seedlings (lower left) show the same
phenotype with inhibition of cotyledon expansion, root growth and
greening as 35S::WUS; 35S::GUS-expressing seedlings (upper left)
when germinated on dexamethasone containing medium, but not on
control medium (lower right). (B) Longitudinal section through a
GUS-stained CLV3::NLSGUS-expressing plant. Staining is restricted
to the stem cells of the SAM, mirroring the CLV3 mRNA expression
pattern (compare with Fig. 2I). (C-J) Light micrographs of GUS-
stained and cleared seedlings. Seedlings in C,E,G,I were treated with
mock solution for 2 days, while seedlings in D,F,H,J were induced
with 5 µM dexamethasone for the same time. (C,D) After
dexamethasone treatment of 35S::WUS-GR; CLV3::NLSGUS
seedlings (D), strong ectopic GUS expression is observed in
cotyledons (c), leaves (l) and hypocotyl (h), mainly associated with
vascular strands, while expression is restricted to the stem cells of the
SAM in uninduced seedlings (arrowhead, C). (E,F) No difference in
the GUS staining pattern is observed between dexamethasone
induced (F) and uninduced (E) 35S::WUS-GR; KNAT1::GUS-
expressing seedlings. (G,H) No difference in the GUS staining
pattern is observed between dexamethasone induced (H) and
uninduced (G) 35S::WUS-GR; KNAT2::GUS-expressing seedlings,
even though the first morphological effects of ectopic WUS activity
on young leaves – reduced expansion of the lamina and upright
position – are already visible (arrowhead). (I,J) Occasional
ectopically staining cells are visible along the vasculature of the first
pair of rosette leaves in dexamethasone-treated 35S::WUS-GR;
CycB1;1::CDBGUS-expressing seedlings (arrowhead in J), which
were never observed in mock-treated seedlings of the same genotype
(arrowhead in I). Scale bars are 5 mm in A, 100 µm in B and 500 µm
in C-J.
ectopic STM activity does not appear to induce expression fromthe WUS promoter.
Taken together these results indicate that ectopic WUS and
STM activities function independently of each other.
Coexpression of WUS and STM produces
synergistic effects
Their loss-of-function phenotypes indicate that both WUS and
STM activities are essential for SAM function (Barton and
Poethig, 1993; Endrizzi et al., 1996; Laux et al., 1996), yet our
above experiments demonstrate that their functions are
genetically independent. One interpretation of these findings is
that the developmental pathways regulated by them ultimately
These observations indicate that suppression of leaf
converge on some downstream process. We thus asked whether
formation by ectopic WUS activity does not require STM and
ectopic WUS and STM functions act synergistically on some
suggest that STM is not an essential downstream target of WUS.
shared process and coexpressed both in developing cotyledons
In the converse experiment, we tested whether WUS might
and leaf primordia. Except for a widening of the cotyledon
be a downstream target of STM. To do so, we analyzed whether
petioles in ANT::STM-expressing plants, ectopic expression of
WUS is required for the effects of ectopic STM activity by
either gene alone under the control of the ANT promoter leaves
expressing ANT::STM in wus1 mutants. ANT::STM-expressing
the cotyledons largely unaffected, although staining for the
wus1 mutant plants exhibited a leaf phenotype that was
activity of a linked ANT::GUS reporter gene showed the
indistinguishable from the effect of ANT::STM expression in a
transgenes to be expressed throughout embryonic cotyledon
wild-type background (Fig. 4G-J), suggesting that WUS is not
primordia (data not shown). By contrast, ANT::STM;
an essential downstream target of ectopically expressed STM.
ANT::WUS coexpressing seedlings, in which the presence of
This finding was confirmed by analyzing the expression of a
both transgenes was confirmed by PCR (data not shown),
WUS::NLSGUS reporter gene in plants with ectopic STM
showed a novel phenotype which was clearly distinct from the
activity. ANT::STM; WUS::NLSGUS plants showed GUS
effects of ectopic expression of either gene alone (Fig. 5A-D):
staining in a small central cell group in the shoot meristem, in
they completely lacked cotyledon petioles and had fields of
a pattern that was indistinguishable from that in wild type (Fig.
small cells extending from the apex into the lamina of the
4K-M), but they did not show ectopic GUS staining in the cells
cotyledons. These cells strongly resembled the dense
that expressed ANT::STM (compare with Fig. 2D). Thus,
meristematic cells in the apex of ANT::WUS plants as judged
WUS and STM in shoot meristem regulation 3201
Fig. 4. Independent functions of WUS
and STM. Light micrographs of live
seedlings (A,E-H) and GUS-stained,
cleared seedlings (B-D,I-L).
(A,B) ANT::WUS; ANT::GUS-
expressing wild-type seedlings 12
days (A) and 10 days (B) after
germination. An enlarged SAM has
developed in place of leaves (A)
which strongly expresses the
transgenes (B). (C-E) ANT::WUS;
ANT::GUS-expressing stm5 mutant
seedlings 10 days (C) and 18 days
(D,E) after germination. Transgene
expression has only been initiated in a
few cells (arrow) inside the fused
cotyledon petioles in the seedling in C
from which a mass of small
meristematic cells develops
subsequently (D,E arrow). In E, the
fused cotyledon petioles have been cut
open for clarity. (F) Non-transgenic
stm5 mutant seedling 18 days after
germination. Several leaves have been
formed and have ruptured the fused
wall of the cotyledon petioles.
(G) ANT::STM-expressing wild-type
seedling. Leaves are reduced to finger-
like, lobed structures (arrow) and the petioles of the cotyledons (c) are broadened. (H) ANT::STM-expressing wus1mutant seedling. Leaves (arrow) and cotyledon petioles (c) are affected as in G. (I,J) ANT::STM; ANT::GUS-expressing wild-type (I) and wus1 mutant (J) seedlings. In both cases, strong GUS staining is visible in thevascular strands of the cotyledon petioles (arrowheads) and in young leaf primordia (arrows) at the shoot meristem.
(K,L) WUS::NLSGUS- (K) and ANT::STM; WUS::NLSGUS- (L) expressing seedlings. In both cases, GUSstaining is restricted to a small central cell group in the shoot apical meristem (arrowheads). The additional smallerregion of staining in K is an axillary meristem. (M) Longitudinal section through a GUS-stained WUS::NLSGUS-expressing seedling. GUS activity is detected specifically in a small central cell group of the SAM, reflecting theWUS mRNA expression pattern (Mayer et al., 1998). Scale bars are 1 mm in A-L, 100 µm in M.
from their appearance under the scanning electron microscope
rescue the mutant phenotype when combined with a WUS
and in histological sections (Fig. 5E,F) and showed ectopic
target line (Groß-Hardt et al., 2002) and was confirmed by
CLV3 expression (Fig. 5G,H).
staining for the activity of a linked CLV1::GUS reporter (Fig.
Thus, simultaneous ectopic expression of WUS and STM
6A,B). The phenotype of CLV1::STM; wus1 plants was
produced a non-additive phenotype in that meristematic cells
indistinguishable from that of non-transgenic wus1 mutants:
were induced in cotyledons which was not the case in
shoot development in seedlings of both genotypes arrested
plants expressing either gene alone. This suggests that in
after the formation of two to three leaves (Fig. 6E,F). 10 days
differentiated tissue both genes synergistically confer meristem
after germination, we observed strong transgene expression in
cell identity.
what are most likely adventitious meristems (Fig. 6D; see Lauxet al., 1996). Despite this, no self-maintaining meristems could
Increased WUS activity can induce self-maintaining
be formed in a wus1 mutant background, and CLV1::STM-
meristems in stm mutants, but not vice versa
expressing wus1 mutant plants showed the same ‘stop and go'
We next asked whether similar to the results of the above
mode of development as non-transgenic wus1 mutants (Laux
ectopic coexpression experiment, the pathways activated by
et al., 1996; data not shown). The leaves, however, showed the
WUS and STM also converge in the regulation of SAM
same wrinkled phenotype that was also observed in
function. We therefore tested whether an increase of one gene's
CLV1::STM-expressing wild-type plants and which appears to
activity in the SAM could compensate for the effects of a
be due to weak expression of the transgene in leaves as judged
mutation in the other gene. For this purpose we expressed WUS
by prolonged staining for the activity of the linked CLV1::GUS
or STM under the control of the CLV1 promoter in the
reporter gene (data not shown), confirming that in principle
respective other mutant.
STM was active in wus mutants.
First, we expressed CLV1::STM in wus1 mutants. Since the
Thus, increasing STM expression in the shoot apex is not
expression patterns of transgenic and endogenous STM roughly
able to compensate for the shoot meristem defects of wus
overlap, this would be expected to increase the STM expression
level throughout the apex. Expression of the CLV1 activator
Secondly, in the converse experiment, we analyzed the
line in wus1 mutant embryos was evident from its ability to
effects of CLV1::WUS
expression in stm5 mutants.
3202 M. Lenhard, G. Jürgens and T. Laux
CLV1::WUS-expressing wild-type seedlings produce anenlarged meristem immediately after germination due to theenlarged WUS expression domain throughout the SAM(Fig. 6G-J) (Schoof et al., 2000). By contrast, 7 days aftergermination stm5 mutant seedlings carrying the CLV1::WUStransgene lacked a recognizable shoot meristem and wereindistinguishable from non-transgenic stm5 mutant seedlings.
That the CLV1 activator was expressed in stm5 mutants wasdemonstrated by its ability to rescue the mutant defect whencombined with an STM target line (see above, Fig. 1A-D);however, even in combination with our strongest WUS targetline, the resulting embryonic expression was only very weakas judged from staining for the activity of a linked CLV1::GUSreporter gene (data not shown). While such weak expressionappears to be sufficient to rescue the wus1 mutant defect(Groß-Hardt et al., 2002), it is apparently unable to overcomethe lack of STM activity during embryogenesis. After day 7,CLV1::WUS; CLV1::GUS-expressing stm5 mutant seedlingsshowed small clusters of GUS staining cells inside the fusedcotyledon petioles and by day 12 after germination, 26 out of40 seedlings had developed a conspicuous adventitiousstructure resembling a meristem surrounded by small leafprimordia (Fig. 6J,K,M,N). No similar structures wereobserved in any of 25 non-transgenic stm5 mutant seedlings 12days after germination (Fig. 6L).
To analyze whether the induced structures were meristems,
we examined them for expression of the meristem markergenes CLV3, KNAT1 and KNAT2 using in situ hybridization(see above). Both CLV1::WUS-expressing wild-type and stm5mutant seedlings 10 or 14 days after germination showedstrong CLV3 expression in the outermost cell layers across theirenlarged meristems and the induced structures, respectively(Fig. 7A,B). By contrast, we could not detect CLV3 expressionin any of 25 non-transgenic stm5 mutant seedlings 10 days aftergermination (data not shown). While we could not detectKNAT1 expression in the induced structures of 10-day oldCLV1::WUS-expressing stm5 mutant seedlings (Fig. 7E,F;
Fig. 5. Synergistic effects of coexpression of WUS and STM.
n=6; see Materials and Methods), by 14 days after germination
(A-D) Scanning electron micrographs of seedlings 14 days after
the induced structures in CLV1::WUS-expressing stm5 mutant
germination. (A) ANT::WUS-expressing seedling. An enlarged SAM
seedlings showed clear KNAT1 expression in small patches on
has formed in place of leaves. The cotyledon petioles (cp) areunaffected and separated from the meristematic cells by a sharp
the flanks and at their base close to the vasculature (Fig. 7G),
boundary (arrow). h, hypocotyl. (B) ANT::STM-expressing seedling.
similar to the pattern observed in meristems of CLV1::WUS-
Cotyledon petioles (cp) are broadened, but do not show meristem-
expressing and non-transgenic wild-type seedlings (Fig. 7C,D)
like cells. (C,D) ANT::WUS; ANT::STM coexpressing seedlings. No
(Chuck et al., 1996). Hybridization with a KNAT2 antisense
cotyledon petioles have been formed and fields of small,
riboprobe produced a similar result: While no KNAT2
meristematic cells (arrows) extend into the lamina of cotyledons (c).
expression could be detected in the induced structures of 10-
(E,F) Histological sections of plastic embedded material stained with
day old CLV1::WUS-expressing stm5 mutant seedlings (Fig.
Toluidine Blue. (E) Longitudinal section through the apex of an
7I; n=11; see Materials and Methods), consistent weak staining
ANT::WUS-expressing seedling 8 days after germination. Note the
was found at the flanks and base of the induced structures by
massively overproliferated shoot meristem with small,
14 days after germination (Fig. 7J). CLV1::WUS-expressing
cytoplasmically dense cells (arrow). (F) Longitudinal section throughthe apex of an ANT::WUS; ANT::STM-expressing seedling 8 days
wild-type seedlings showed virtually the same expression
after germination. The regions of small meristematic cells are
pattern for KNAT2 as found for KNAT1, i.e. at the periphery of
expanded into the cotyledons (arrows). The spots of darker stained
the enlarged SAM and at the base of young leaf primordia (Fig.
cells are an artefact of processing. (G,H) In situ hybridization using a
CLV3 antisense riboprobe. (G) In ANT::WUS-expressing seedlings,
Thus, the structures induced by CLV1::WUS expression in
CLV3 mRNA is detected in the outermost cell layers of the enlarged
stm5 mutant seedlings showed expression of the three marker
shoot meristem (black arrow), but not in cells of the cotyledon
genes tested, suggesting that they represent meristems.
petioles (white arrow). (H) By contrast, ANT::WUS; ANT::STM
However, these meristems never reached a size comparable to
coexpressing seedlings show CLV3 expression both in the enlarged
those formed by CLV1::WUS-expressing wild-type plants, as
shoot meristem (black arrow) and in the meristematic regions on the
judged from staining for the activity of the linked CLV1::GUS
cotyledons (white arrow). Scale bars are 500 µm in A-C, 200 µm inD and 100 µm in E-H.
reporter gene (Fig. 6O,P). Since the size of the cells in
WUS and STM in shoot meristem regulation 3203
Fig. 6. The loss-of-function phenotypes of
wus and stm mutants cannot be rescued by
transgenic expression of the respective other
gene. Light micrographs of GUS stained
cleared embryos or seedlings (A-D,G,J-
L,O,P) and of live seedlings (E,F,H,M,N).
(A,B) The CLV1::STM transgene is strongly
expressed in the SAM primordia (arrows) of
wild-type (A) and wus1 mutant (B) embryos
as indicated by staining for the activity of a
linked CLV1::GUS reporter. Note the flat
apex of the wus1 mutant embryo compared
to the convex meristem in the wild type,
suggesting that the former has terminated.
(C) CLV1::STM; CLV1::GUS expression is
detected in the SAM of 7-day old wild-type
seedlings by GUS staining. (D) CLV1::STM;
CLV1::GUS-expressing wus1 mutant
seedlings 10 days after germination show
strong GUS staining at the shoot apex.
(E,F) The meristems in CLV1::STM;
CLV1::GUS-expressing wus1 mutant
seedlings (F) terminate indistinguishably
from the meristems in non-transgenic wus1
mutants (E) (arrows). (G,H) In CLV1::WUS;
CLV1::GUS-expressing wild-type seedlings
7 days after germination strong GUS
staining is detected at the apex (G) which
causes the development of an enlarged
meristem (H, arrow). (I) In situ
hybridization using a WUS antisense
riboprobe on CLV1::WUS-expressing
seedlings confirms transgene expression
specifically in the centre of the enlarged
shoot meristem, yet not on the flanks (arrow)
where organs are initiated. (J,K) In CLV1::WUS; CLV1::GUS-expressing stm5 mutant seedlings the first GUS-staining cells are detected 7 days
after germination inside the fused cotyledon petioles (arrow in J) which give rise to adventitious meristems (K, compare with M,N).
(L-N) While non-transgenic stm5 mutants 12 days after germination show no sign of a SAM inside the fused cotyledon petioles (L),
CLV1::WUS-expressing stm5 mutant seedlings (M,N) of the same age contain a conspicuous meristematic structure (arrows) that is surrounded
by small leaf primordia (arrowhead in N). (O) In CLV1::WUS; CLV1::GUS-expressing wild-type plants 25 days after germination, the
meristem is massively enlarged (arrow). (P) CLV1::WUS; CLV1::GUS-expressing stm5 mutant plants of the same age show only small
meristematic regions that express the GUS reporter gene (arrow). In addition, leaves are small and sometimes fused as in non-transgenic stm5
mutant plants. Scale bars are 50 µm in A,B, 1 mm in C-H,J-P, and 100 µm in I.
CLV1::WUS-expressing wild-type and stm5 mutant meristems
same processes, formation and maintenance of a functional
appeared to be roughly equal (compare Fig. 7A and 7B), the
shoot meristem (Barton and Poethig, 1993; Endrizzi et al.,
reduced growth of the meristem in stm5 seedlings likely results
1996; Laux et al., 1996), yet it is unknown whether and how
from fewer cell divisions, rather than from reduced cell
their functions are integrated in SAM regulation. To address
expansion. This suggests a critical requirement for STM in
this issue, we have analyzed their genetic interactions using a
allowing amplification of meristem cells which cannot be
combination of gain- and loss-of-function experiments.
compensated for by increased WUS activity.
In summary, CLV1::STM expression in wus mutants cannot
STM and WUS function in different pathways in
compensate for the loss of WUS function. However, conversely
shoot meristem regulation
expressing CLV1::WUS in stm mutants induces the formation
Our results suggest that WUS and STM fulfil independent, yet
of adventitious shoot meristems at a high frequency, although
complementary functions in SAM regulation, for the following
it cannot fully rescue the stm mutant defect. Thus, it appears
that increasing WUS activity can at least partly compensate for
(1) When expressed ectopically in leaf primordia, the
the loss of STM function, suggesting a convergence of the
effects of WUS and STM are clearly distinct. WUS is sufficient
pathways activated by WUS and STM in SAM regulation.
to completely abolish organ formation, but has little, if any,stimulating effect on cell division, as evidenced both by itsinability to efficiently induce expression of the mitotic marker
gene CyclinB1;1 and by the low proportion of cells in S-phasein the enlarged central zone of CLV1::WUS-expressing
The WUS and STM homeobox genes are both essential for the
meristems (M. L. and T. L., unpublished). By contrast, ectopic
3204 M. Lenhard, G. Jürgens and T. Laux
Fig. 7. Marker gene expression in CLV1::WUS-expressing wild-type
and stm5 mutant plants. Longitudinal sections hybridized in situ with
CLV3 (A,B), KNAT1 (C,D,F,G), KNAT2 (H-J) antisense and KNAT1
sense (E) riboprobes. CLV3 and KNAT2 sense riboprobes did not
produce any staining (not shown). (A) In CLV1::WUS-expressing
wild-type plants 14 days after germination, cells in the three
outermost layers of the meristem show strong CLV3 expression.
(B) CLV1::WUS-expressing stm5 mutant plants 14 days after
germination exhibit CLV3 expression in a band at the top of the
induced structure inside the fused cotyledon petioles. The same
result was obtained when analyzing 10 day old seedlings (not
shown). (C) In non-transgenic wild-type seedlings, KNAT1
expression is detected at the base and periphery of the SAM and
close to the base of young leaf primordia (black arrow), but is absent
from the central zone of the SAM (white arrow). In addition,
expression is detected in cells close to the vasculature (arrowhead).
(D) In CLV1::WUS-expressing wild-type plants 10 days after
germination, KNAT1 expression is detected at the periphery of the
enlarged meristem (black arrows) and adjacent to the vasculature
(arrowhead). Although weak, this staining was consistent throughout
serial sections. The central region of the meristem (white arrow)
shows only weak background staining that is also found in leaves
(asterisk) and in sections hybridized with a KNAT1 sense probe
(compare with E). (E) Hybridization with a KNAT1 sense riboprobe
produces only weak non-specific staining. (F,G) While no KNAT1
mRNA can be detected in the induced structures of 10 day old
CLV1::WUS-expressing stm5 mutant seedlings (F), plants of the
same genotype at 14 days after germination (G) exhibit clear KNAT1
expression at the base (arrow) and in patches on the flanks of the
induced structures (arrowhead). However, no expression is seen close
to the vasculature in either seedling. (H) KNAT2 mRNA can be
detected in the periphery of the enlarged meristem of CLV1::WUS-
expressing wild-type plants 14 days after germination (black arrows),
while only weak and even staining is visible in the centre of the
meristem (white arrow) and in leaves (asterisk) which most likely
represents non-specific background staining. (I,J) In 10-day old
CLV1::WUS-expressing stm5 mutant seedlings (I), no KNAT2
expression can be detected, which is however seen in seedlings of the
same genotype 14 days after germination (J) on the flanks (arrow)
and at the base (arrowhead) of the induced structure. The asterisk in I
indicates a fragment of the vasculature which appears darker because
of its secondary cell wall. Scale bars in A-J are 100 µm.
which has, however, no effect on CLV3 expression. Theconclusion that, unlike WUS, STM thus does not appear to bedirectly involved in stem cell specification is further supportedby our preliminary result that CLV3 expression is initiated in
STM activity still allows organs to develop, but cell
the apex of stm5 mutant embryos, and is lost only in late stages
differentiation is suppressed and the cells continue to
of embryogenesis when the apex differentiates (M. L. and T.
proliferate. This effect is strikingly similar to the phenotype
L., unpublished).
of dominant mutations in knotted1, the maize ortholog of
(3) The gain-of-function phenotypes of ectopic WUS and
STM, whose misexpression in leaves leads to local
STM expression in leaf primordia do not require the activity of
overproliferation (Smith et al., 1992). At least on the basis of
the respective other gene, indicating that they function in
expression levels of the linked GUS reporter genes (Fig. 4B,I),
independent genetic pathways.
these distinct effects do not appear to be due to strongly
(4) The shoot meristem defects of both WUS and STM loss-
differing levels of transgene expression, suggesting that they
of-function mutants cannot be rescued by transgenic
reflect intrinsic functional differences between the two
expression of the other gene: transgenic expression of STM in
transcription factors.
the apex is not able to compensate for the lack of self-
(2) Ectopic expression of WUS and STM in leaf primordia
maintaining stem cells in wus mutants. Conversely, even
induces the expression of distinct downstream target genes.
though WUS expression can induce the formation of meristems
WUS is able to induce expression of the presumed stem cell
in stm mutants, these appear to grow significantly slower than
marker CLV3 even in differentiated organs, but does not
the corresponding meristems in a wild-type background,
activate KNAT1 or KNAT2 expression. By contrast, expression
suggesting that loss of STM function results in reduced
of the latter genes can be induced by ectopic STM activity,
proliferation of meristem cells and/or their premature
WUS and STM in shoot meristem regulation 3205
differentiation. Thus, WUS and STM appear to fulfil distinct
frequent meristem initiation? One conceivable interpretation
functions in shoot meristem regulation.
is that meristems can be formed as long as there are enough
(5) Based on the synergistic effect of ectopically
undifferentiated cells, no matter whether these are produced
coexpressing both genes in leaf primordia and on the ability of
by increasing the size of the WUS expression domain – as in
WUS to partly compensate for loss of STM activity in the
CLV1::WUS-expressing plants or in clv loss-of-function
apex, the developmental pathways regulated by WUS and
mutants – or by a small WUS-expressing region in
STM appear to converge, in that both genes suppress cell
combination with STM activity in a larger zone as in the wild-
type apex. In contrast to WUS, STM on its own does not appearto be able to induce self-maintaining meristems in the absence
Integration of WUS and STM in shoot meristem
of WUS function. This could either be due to a reduced
potency of STM in suppressing differentiation compared to
Our data suggest the following model for how the independent
WUS or to its inability to induce stem cells, which are lacking
pathways regulated by WUS and STM are integrated to produce
in wus mutants, or to a combination of both. Differences
a self-maintaining meristem. In the central region of the
between the two genes in their potency to suppress cell
meristem WUS-dependent signalling from the organizing
differentiation are suggested by the different severity of the
centre specifies an apical stem cell niche whose residents act
effects caused by ectopic expression of WUS or STM in leaf
as long-term stem cells. STM is not directly involved in stem
cell specification, but is required throughout the meristem
Evidence supporting the above hypothesis that formation of
dome to antagonize cell differentiation and allow meristem
a stable SAM requires a critical number of undifferentiated
cells to proliferate. Thus, peripheral stem cell daughters are
cells has also been obtained by studying the STM ortholog
prevented from being prematurely incorporated into organ
KNOTTED1 in maize (Vollbrecht et al., 2000). The penetrance
anlagen and can amplify cell numbers. STM appears to act by
of the meristem defect in knotted1 mutant embryos is inversely
repressing AS1 expression and thus allowing expression of the
correlated with the size of the meristem primordium in wild-
homeobox genes KNAT1 and KNAT2 (Byrne et al., 2000).
type embryos of the respective genetic background, such that
Local downregulation of STM expression in the periphery
knotted1 mutants form meristems much more frequently in
finally allows lateral organs to be formed.
inbred lines with a large meristem primordium than in ones
The observations described here and in previous studies
with a small meristem primordium.
(Mayer et al., 1998; Fletcher et al., 1999) suggest a refinement
Secondly, meristem initiation appears to depend on a
of the classical histological zonation concept of the SAM
competence of cells to switch to meristem identity, which they
(Steeves and Sussex, 1989). The centre of the shoot meristem,
appear to gradually lose as they differentiate. While relatively
roughly equivalent to the central zone, is composed of an apical
undifferentiated cells in leaf anlagen can easily be respecified
stem cell niche, whose residents express the CLV3 gene,
towards stem cell identity by WUS alone, the differentiated
and the underlying WUS-expressing organizing centre. The
cells in cotyledons are no longer responsive to WUS alone.
peripheral zone comprises a transition zone, where
However, this block to switch to meristem identity can be
differentiation is repressed by STM, allowing the cells to
overcome by the combined effects of WUS and STM,
amplify, and regions where STM expression is discontinued
suggesting that a strongly reduced cellular competence can be
and organ primordia are initiated.
compensated for by increased meristem promoting activity.
Similar to other stem cell systems (Potten and Loeffler,
This synergistic effect of coexpressing WUS and STM could
1990), the amplification of cell numbers by the peripheral stem
have important biotechnological implications for adventitious
cell daughters may allow the long-term stem cells to divide
meristem formation from differentiated cells, which could
only relatively rarely – for example only once per 14 initiated
possibly be strongly enhanced by coexpression of WUS and
leaves in privet (Stewart and Dermen, 1970), while still
ensuring a continuous supply of sufficient cells for organ
In summary, the results presented here indicate that WUS
initiation. This division of labour could in turn minimize the
and STM serve distinct functions in the SAM, regulation of
danger for stem cells of incurring mutations associated with
stem cell identity and protection of meristem cells from
DNA replication and chromosome segregation. As a large
premature differentiation, respectively, and support a division
portion of the plant body is ultimately derived from a single
of labour between a slowly dividing set of long-term stem cells
stem cell (Stewart and Dermen, 1970), mutations in them
and a more rapidly proliferating population of stem cell
would likely be more deleterious than mutations in their
daughters that only transiently function as initials, both of
daughter cells which only give rise to a more limited part of
which are required for continuous organ formation from a self-
A critical number of cells and cellular competence
We would like to thank R. Williams for providing the
appear to be required for shoot meristem initiation
construct, S. Hake, J. Traas and J. Celenza for
Our results imply two important requirements for meristem
providing KNAT1::GUS, KNAT2::GUS and CycB1;1::CDBGUSlines, respectively, V. Pautot and J. Traas for the KNAT2 plasmid and
formation. First, we found that a CLV1::WUS transgene can
I. Moore for the components of the pOpL expression system. We are
induce adventitious meristems at a high frequency in stm
grateful to Andrea Bohnert for technical assistance and to Arp
mutant seedlings, which is observed to a similar extent in stm
Schnittger and members of the Laux laboratory for helpful
clv double mutants (Clark et al., 1996). In both cases, the
suggestions on the manuscript. This work was supported by grants
effect is likely due to WUS being expressed in an enlarged
from the Deutsche Forschungsgemeinschaft to T. L. and a PhD
domain (Schoof et al., 2000). How might this lead to more
fellowship of the Boehringer Ingelheim Fonds to M. L.
3206 M. Lenhard, G. Jürgens and T. Laux
gametophyte development is related to the floral homeotic gene APETALA2.
Plant Cell 8, 137-158.
Barton, M. K. and Poethig, R. S. (1993). Formation of the shoot apical
Koncz, C. and Schell, J. (1986). The promoter of the TL-DNA gene 5 controls
meristem in Arabidopsis thaliana: an analysis of development in the wild
the tissue-specific expression of chimaeric genes carried by a novel type of
type and in the shoot meristemless mutant. Development 119, 823-831.
Agrobacterium binary vector. Mol. Gen. Genet. 204, 383-396.
Becker, D., Kemper, E., Schell, J. and Masterson, R. (1992). New plant
Laux, T., Mayer, K. F. X., Berger, J. and Jürgens, G. (1996). The
binary vectors with selectable markers located proximal to the left T-DNA
WUSCHEL gene is required for shoot and floral meristem integrity in
border. Plant Mol. Biol. 20, 1195-1197.
Arabidopsis. Development 122, 87-96.
Brand, U., Fletcher, J. C., Hobe, M., Meyerowitz, E. M. and Simon, R.
Lincoln, C., Long, J., Yamaguchi, J., Serikawa, K. and Hake, S. (1994). A
(2000). Dependence of stem cell fate in Arabidopsis on a feedback loop
knotted1-like homeobox gene in Arabidopsis is expressed in the vegetative
regulated by CLV3 activity. Science 285, 585-587.
meristem and dramatically alters leaf morphology when overexpressed in
Byrne, M. E., Barley, R., Curtis, M., Arroyo, J. M., Dunham, M., Hudson,
transgenic plants. Plant Cell 6, 1859-1876.
A. and Martienssen, R. A. (2000). Asymmetric leaves1 mediates leaf
Long, J. A., Moan, E. I., Medford, J. I. and Barton, M. K. (1996). A
patterning and stem cell function in Arabidopsis. Nature 408, 967-971.
member of the KNOTTED class of homeodomain proteins encoded by the
Chuck, G., Lincoln, C. and Hake, S. (1996). KNAT1 induces lobed leaves
STM gene of Arabidopsis. Nature 379, 66-69.
with ectopic meristems when overexpressed in Arabidopsis. Plant Cell 8,
Long, J. A. and Barton, M. K. (1998). The development of apical embryonic
pattern in Arabidopsis. Development 125, 3027-3035.
Clark, S. E., Running, M. P. and Meyerowitz, E. M. (1993). CLAVATA1, a
Mayer, K. F. X., Schoof, H., Haecker, A., Lenhard, M., Jürgens, G. and
regulator of meristem and flower development in Arabidopsis. Development
Laux, T. (1998). Role of WUSCHEL in regulating stem cell fate in the
Arabidopsis shoot meristem. Cell 95, 805-815.
Clark, S. E., Running, M. P. and Meyerowitz, E. M. (1995). CLAVATA3 is
Mironov, V., de Veylder, L., van Montagu, M. and Inzé, D. (1999). Cyclin-
a specific regulator of shoot and floral meristem development affecting the
dependent kinases and cell division in plants – the nexus. Plant Cell 11,
same processes as CLAVATA1. Development 121, 2057-2067.
Clark, S. E., Jacobsen, S. E., Levin, J. Z. and Meyerowitz, E. M. (1996).
Moore, I., Gälweiler, L., Grosskopf, D., Schell, J. and Palme, K. (1998). A
The CLAVATA and SHOOT MERISTEMLESS loci competitively regulate
transcription activation system for regulated gene expression in transgenic
meristem activity in Arabidopsis. Development 122, 1565-1575.
plants. Proc. Natl. Acad. Sci. U.S.A. 95, 376-381
Clark, S. E., Williams, R. W. and Meyerowitz, E. M. (1997). The CLAVATA1
Neff, M. M., Neff, J. D., Chory, J. and Pepper, A. E. (1998). dCAPS, a
gene encodes a putative receptor-kinase that controls shoot and floral
simple technique for the genetic analysis of single nucleotide
meristem size in Arabidopsis. Cell 89, 575-585.
polymorphisms: experimental applications in Arabidopsis thaliana genetics.
Clough, S. J. and Bent, A. F. (1998). Floral dip: a simplified method for
Plant J. 14, 387-392.
Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J.
Pautot, V., Dockx, J., Hamant, O., Kronenberger, J., Grandjean, O.,
16, 735-743.
Jublot, D. and Traas, J. (2001). KNAT2: Evidence for a link between
Dockx, J., Quaedvlieg, N., Keultjes, G., Kock, P., Weisbeek, P. and
Knotted-like genes and carpel development. Plant Cell 13, 1719-1734.
Smeekens, S. (1995). The homeobox gene ATK1 of Arabidopsis thaliana is
Potten, C. S. and Loeffler, M. (1990). Stem cells: attributes, cycles, spirals,
expressed in the shoot apex of the seedling and in flowers and inflorescence
pitfalls and uncertainties. Lessons for and from the crypt. Development 110,
stems of mature plants. Plant Mol. Biol. 28, 723-737.
Doerner, P., Jorgensen, J. E., You, R., Steppuhn, J. and Lamb, C. (1996).
Sablowski, R. W. and Meyerowitz, E. M. (1998). A homolog of NO APICAL
Control of root growth and development by cyclin expression. Nature 380,
MERISTEM is an immediate target of the floral homeotic genes
APETALA3/PISTILLATA. Cell 92, 93-103.
Endrizzi, K., Moussian, B., Haecker, A., Levin, J. and Laux, T. (1996). The
Satina, S., Blakeslee, A. F. and Avery, A. (1940). Demonstration of three
gene is required for maintenance of
germ layers in the shoot apex of Datura by means of induced polyploidy in
undifferentiated cells in Arabidopsis shoot and floral meristems and acts at
periclinal chimeras. Am. J. Bot. 27, 895-905.
a different regulatory level than the meristem genes WUSCHEL and
Schoof, H., Lenhard, M., Haecker, A., Mayer, K. F. X., Jürgens, G. and
ZWILLE. Plant J. 10, 967-979.
Laux, T. (2000). The stem cell population of Arabidopsis shoot meristems
Elliott, R. C., Betzner, A. S., Huttner, E., Oakes, M. P., Tucker, W. Q.,
is maintained by a regulatory loop between the CLAVATA and WUSCHEL
Gerentes, D., Perez, P. and Smyth, D. R. (1996). AINTEGUMENTA, an
genes. Cell 100, 635-644.
APETALA2-like gene of Arabidopsis with pleiotropic roles in ovule
Smith, L. G., Greene, B., Veit, B. and Hake, S. (1992). A dominant mutation
development and floral organ growth. Plant Cell 8, 155-168.
in the maize homeobox gene, Knotted-1, causes its ectopic expression in
Fletcher, J. C., Brand, U., Running, M. P., Simon, R. and Meyerowitz, E.
leaf cells with altered fates. Development 116, 21-30.
M. (1999). Signaling of cell fate decisions by CLAVATA3 in Arabidopsis
Steeves, T. A. and Sussex, I. M. (1989). Patterns in Plant Development.
shoot meristems. Science 283, 1911-1914.
Cambridge, UK: Cambridge University Press.
Groß-Hardt, R., Lenhard, M. and Laux, T. (2002). WUSCHEL signalling
Stewart, R. N. and Dermen, H. (1970). Determination of number and mitotic
functions in interregional communication during Arabidopsis ovule
activity of shoot apical initial cells by analysis of mericlinal chimeras. Am.
development. Genes Dev. (in press).
J. Bot. 57, 816-826.
Jeong, S., Trotochaud, A. E. and Clark, S. E. (1999). The Arabidopsis
Vollbrecht, E., Reiser, L. and Hake, S. (2000). Shoot meristem size is
CLAVATA2 gene encodes a receptor-like protein required for the stability of
dependent on inbred background and presence of the maize homeobox gene,
the CLAVATA1 receptor-like kinase. Plant Cell 11, 1925-1934.
knotted1. Development 127, 3161-3172.
Klucher, K. M., Chow, H., Reiser, L. and Fischer, R. L. (1996). The
Williams, R. W. (1998). Plant homeobox genes: many functions stem from a
AINTEGUMENTA gene of Arabidopsis required for ovule and female
common motif. BioEssays 20, 280-282.
Source: http://www.biologie.uni-freiburg.de/data/bio3/laux/publications_files/Lenhard2002.pdf
wacanaseni.usm.my
Yasmin Ahmad: Auteuring a New Malaysian Cinematic Landscape Lee Yuen Beng School of Communication, Universiti Sains Malaysia, MALAYSIA Since P. Ramlee, no other filmmaker but Yasmin Ahmad has been capable of creating a significant impact in Malaysian cinema. She achieved this through her films that have persistently challenged not only the conventions of Malaysian cinema, but also daringly
Chapter 10: supportive care
CHAPTER 10: SUPPORTIVE CARE Lung cancer and its treatment can cause many symptoms that may interfere with your ability to live as you normally would. Health care providers often refer to this interference as a reduction in quality of life or QOL. Supportive care is a term that refers to treatments used to eliminate or reduce symptoms that interfere with your quality of life. The aim of supportive care is to provide you with the best quality of life possible, so that you are able to participate