Polyplatillen.com.ua
Ministry of Healthcare of UkraineUkrainian Center of Scientific Medical Information and Patent Licensing Activity
Use of the National Antineoplastic Drug of Platinum on DNA carrier at Treatment
of the Advanced Forms of Malignant Neoplasms
Kiev – 2010
Institution-Developer: SE «National Cancer Institute» MHC of Ukraine
Institution-Codeveloper: Medical and Preventive Treatment Facility Donetsk Regional
Anti Cancer Center
Authors:
Dudnichenko Alexander Sergeyevitch – Doctor of Medical Sciences, professor;
Vorobyov Oleg Nickolayevitch – Candidate of Medical Sciences;
Lischishina Elena Mikhailovna – Candidate of Medical Sciences;
Lisovskaya Natalia Yurievna – Candidate of Medical Sciences;
Komendant Vasiliy Vasilyevitch;
Martsenkovskaya Natalia Vadimovna.
Contact number: (062) 223-89-85
Reviewer: Sedakov Igor Yevgenyevitch – Doctor of Medical Sciences, professor.
Chairman of the Task Group «Oncology»
AMS and MHC of Ukraine: Bondar Grigoriy Vasilyevitch – Doctor of Medical
Sciences, professor, academician of the AMS of Ukraine.
CONTENT
Introduction 5
1. Pharmacological characteristics of the drug 7
2. Features of the mode of action 7
3. Results of patients treatment in retrospective.
Conclusions of randomized research and clinical experience 8
4. Indications, contra-indications, mode of administration and doses 14
5. Regimens of polychemotherapy 15
6. Conclusions 19
7. List of the reference literature 20
THE LIST OF ABBREVIATIONS
PPL – Polyplatillen
LC – lung cancer
SC – stomach cancer
PC – pancreas cancer
CC – colorectal cancer
BT – beam therapy
CT – standard chemotherapeutic methods of treatment
Surgery – surgical methods of treatment
Timely diagnostics and treatment of malignant neoplasms plays a considerable role in
preservation of health of the population of Ukraine. Number of patients who were
diagnosed with «malignant tumor» is approaching 1 million. Annually doctors define
over 160000 of new cases.
Urgency of the problem of rendering of the adequate specialized help to the patients
aggravates due to a high proportion of the newly-diagnosed patients with advanced
stages of malignant neoplasms – on the average up to 40 %. Within a year from the
moment of making a diagnosis lethality makes 35 %. About 66 % of newly-diagnosed
patients in Ukraine get antineoplastic treatment, including exclusively surgical treatment
– 34 % combined and complex treatment – no more than 32 %.
Today achievement of successes in treatment of oncological pathology is in many
respects conditioned by a very rapid development of chemotherapy in comparison with
other methods of antineoplastic intervention. New antineoplastic medications are being
implemented, treatment regimens are being improved, possibilities are being extended
and
The real breakout in chemotherapy of malignant tumors is application of platinum
medications which till now remain the basic component of the majority of modern
protocols and treatment regimens. At the same time absence of selective effect on a
tumor leads to development of side effects and complications. Accordingly different
organs and systems are malfunctioning that additionally raises endotoxicosis of
oncological patients and negatively influences the quality of the patients' life.
Modern platinum drugs show high antineoplastic activity, but at the same time nephro -
hemato - and other kinds of toxicity.
It is toxicity that owing to chemotherapy in most cases usually limits full range
treatment that leads to initiation of the further research of new antineoplastic drugs with
strengthening its selective effect on a tumor and decreasing toxicity concerning healthy
tissues.
Creation of platinum compound with biopolymer became a defining step in the solution
of the specified above problems. Immobilization of cis-isomer of
dihlorodiamminplatinum into exogenous high-molecular DNA caused considerable
decrease in the general toxicity and also development of tumors resistance to
platiniferous cytostatics.
Polyplatillen – chemical name poly(hexacos-[chlorideammineaqua-platinum(II)])-μ-
deoxyribonucleate is a compound of cis-isomer of dihlorodiamminplatinum with the
high-molecular carrier – deoxyribonucleic acid (DNA), created by national scientists.
(The patent for the invention № 86338 "Antitumor agent based on platinum compound
with DNA and method of its production" is registered in the State register of patents of
Ukraine on inventions on 4/10/2009).
Randomized clinical research was carried out on three clinical bases:
1) Kyiv Municipal Oncological Hospital.
Abdominal surgery department.
69, Verhovinnaja Str., Kiev;
2) Institute of Oncology of the AMSU.
Department of general oncology and premalignancies.
33/43, Lomonosov Str., Kiev;
3) Chair of Facultative Surgery of the Lviv State Medical University of Daniel Galitsky.
Lviv.
(Total number of patients who took part in a randomized clinical research is 1036
patients with LC, SC and PC).
Methodological recommendations present a new solution to the increase of efficiency of
chemotherapy of advanced and resistant malignant neoplasms with the use of innovative
national antineoplastic drug – Polyplatillen, on the basis of multicenter randomized
clinical research taking into account the requirements of evidentiary medicine.
The invention of Polyplatillen medication was distinguished by the Diploma of the
winner of the All-Ukrainian competition "Invention-2009" in a special nomination "For
development of new technologies in pharmaceutics".
Methodological recommendations are meant for oncologists and experts who are
engaged in organizing the fight against cancer.
Methodological recommendations are prepared in Ukraine for the first time.
1. Pharmacological characteristics of the drug
Pharmacological group of the drug is antineoplastic means, platinum compounds, the
international code – ATC L01XA05.
Polyplatillen chemical name is poly(hexacos-[chlorideammineaqua-platinum(II)])-μ-
deoxyribonucleate.
PPL is produced in the form of a sterile clear solution (a concentrate to prepare solution
for infusions), packed by 250 ml in glass bottles. 1 ml of the concentrate solution
contains 1.47 mg of the active substance.
Storage conditions: PPL should be stored in a dark place at temperature +15 – +25 C°,
shelf life – 2 years. Sterility of the depressurized drug in the concentrate is 7 days, the
increase in opalescence degree is acceptable.
It is not recommended to freeze the drug.
No PPL antidote was found.
2. Features of the mode of action
Selectivity of PPL action on tumor cells was raised due to covalent binding of the active
substance – platinate with exogenous high-molecular DNA.
Property of fast proliferating tumor cells to engage substantially exogenous DNA leads
to the maximum accumulation of an active component of the drug in a tumor.
PPL causes morphological changes in the cell structure damaging permanently the cells
which are at G1-S phase of a cell cycle, through the mechanisms of apoptosis and
necrosis that finally leads to permanent destruction of malignant cells.
The main links of the mode of action of the drug are connected with the presence of
ions of platinum on DNA-carrier which damage DNA, RNA, ATF-phase and tubulin
molecules – the major biological objects of tumor cells.
The PPL mode of action is caused by its ability to get into a cell with its subsequent
translocation through plasmalemma with the subsequent distribution in cytoplasm, and
also through phagocytosis with transformation of macromolecules in phagolysosomes.
The further destiny of the drug in tumor cells can be various: concentration of
derivatives in chromatin with the subsequent cells apoptosis, PPL accumulation in
cytoplasm compartments and formation of large autophagic vacuoles, and also diffuse
scattering in cytoplasm. PPL presence in nuclear chromatin structure is a morphological
confirmation of the influence of this chemomedication on the genetic apparatus of the
malignant neoplasms cells.
Being released from platinum ions, DNA molecules can take part in various stages of an
exchange of nuclear acids of the recipient, including integration into his genome,
strengthening PPL antineoplastic effect.
PPL shows antineoplastic properties due to its ability to brake nuclear DNA synthesis,
permanently damages first of all the cells which are in S-phase of the cycle. High
antineoplastic activity and reduction of a tumor size is marked already at the first
administration of PPL, thus there is no damage of healthy tissues. The medication is
effective at therapy of tumors with the acquired medical resistance to another
chemomedications.
After PPL infusion concentration-time pattern of the drug in blood plasma is
characterized by two phases: the initial phase with the effective half-life 6.4 hours and a
wash-out phase – 72 hours.
After a single infusion the average percentage value of platinum which is washed out
with urine within 2 days with the intervals of 0-12, 0-24 and 0-48 hours makes
approximately 20 % of the administered dose of platinum that indicates considerable
not-renal clearance. PPL is washed out through a digestive tract and, unlike others
chemomedications, does not show considerable nephrotoxic effect.
3. Results of patients treatment in retrospective. Conclusions of randomized
research and clinical experience.
High antineoplastic activity of PPL at relatively smaller toxicity in comparison with
antineoplastic medications which are applied at standard schemes of chemotherapy is
proved in clinical conditions.
Low hemato-, nephro-, oto-, neurotoxicity of PPL was determined that has provided a
possibility of carrying out chemotherapeutic treatment of the patients to whom the
standard antineoplastic therapy is counter-indicative.
Efficiency of PPL application for the patients with advanced forms of LC, SC and PC is
confirmed by the results of morphological research, according to which PPL in a
therapeutic dose does not cause permanent structural changes of internal organs,
showing the expressed antineoplastic property.
Morphological research at ultrastructural level testifies to penetration of molecules of
the platinum medication into cytoplasm and nucleus of tumor cells that leads to their
permanent damage (pathological mitoses, apoptosis).
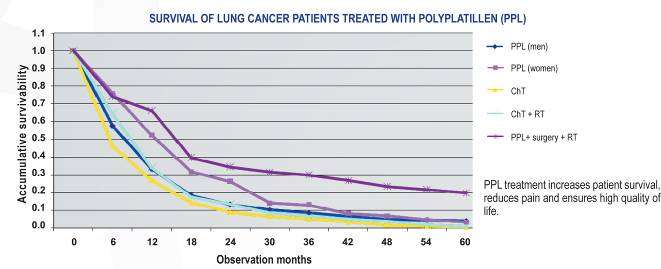
1. PPL positive influence on the survival rate of the patients with the advanced forms of
LC was noted: the maximum life expectancy of the patients was 40 months; the average
life expectancy (ALE) – 10.3 ± 0.8 months; with a median – 8.0 ± 0.8 months; for the
patients treated under the standard regimens the corresponding indicators were lower
and made 22 months, 6.1 ± 0.2 months ± 1 month, 5.0 ± 0.2 months (р <0.05). The
established antineoplastic sensitivity
of non-small-cell lung cancer to PPL therapy testifies to the advisability of its
application in the treatment regimens of such patients.
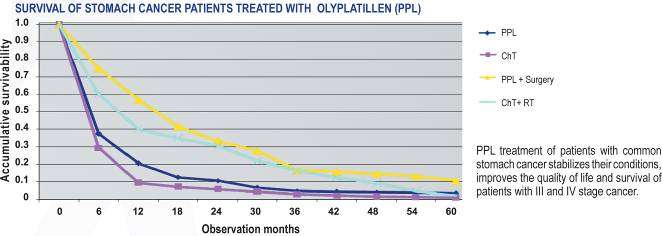
2. PPL application for the patients with the advanced forms of SC without ascites
presence significantly increases the average life expectancy by 11.8 months (р<0.05),
with ascites — by 4.2 months (<0.05) in comparison with the reference group. The
patients with ascites manifestations showed the decrease of accumulation or absence of
ascitic liquids at 30 % of cases when treated with cisplatin and at 85 % of cases when treated with PPL. 3. At PPL application for treatment of the patients with the advanced forms of PC the increase in the average life expectancy by 2.9 months is marked, the survival rate medians by 1.5 months, the maximum life expectancy by 10 months in comparison with the reference group (р <0,05) is marked. According to the data of the National cancer-register of Ukraine for 2010, 1510 of the patients were treated with PPL who had the advanced forms of cancer mainly on III-IV stages of oncological process. All patients were given from 3 to 6 courses of chemotherapy. As a result of treatment due to application of PPL medication it was possible to achieve the objective effect (full and partial remission) at 55 % of patients, at 35 % – the stabilization of the oncological process and to prevent metastatic damage of organs and systems.
Results of treatment of the patients with the advanced LC
At treatment of the patients with LC at IIIВ-IV stages with PPL application the high survival rate of 3 and 5 years was achieved. Below is a table of Kaplan-Meier estimates of survival rate for the patients with LC treated with PPL.
PPL application in the monochemotherapy mode and in combination with other chemomedications allowed us to reach statistically significant improvement of the results of treatment of the advanced forms of LC. At inclusion of beam therapy in PPL treatment of the patients the tendency of increase of efficiency indicators of treatment of the patients with LC is revealed. Combination of the various chemotherapeutic means influencing various molecular targets of a tumor cell allows to overcome effectively the radio resistance of a tumor and to increase a general antineoplastic effect. In this connection the expansion of a spectrum of polychemotherapy regimens on PPL basis in combination with beam therapy to increase the treatment efficiency is necessary. At the same time the expressiveness of PPL medical effect did not depend on a minute
structure of tumors, the drug had almost identical antineoplastic effect on the patients
both with small-cell and non-small-cell LC.
Character of antineoplastic action of PPL on the patients with the advanced forms of
SC
At PPL treatment of the patients with SC at III-IV stage, at 85 % of them who had
ascites intraperitoneal administration of the drug caused significant reduction or
disappearance of accumulation of ascitic fluid.
PPL application at treatment of the patients with the advanced forms of SC positively
improves the quality of life, stabilizes the status of the patient, improves life expectancy
indicators of the patients at III-IV disease stages.
The results of adjuvant therapy of SC supplement the data on the efficiency and safety
of PPL medication and besides show PPL efficiency at ascitic forms of SC,
carcinomatoses and polyserosites.
Improvement of the life quality of the patients is preconditioned by high medical effect
of PPL and low toxicity. The results of ultrastructural changes in SC cells after
chemotherapy neoadjuvant courses is the evidence to this. These researches were
carried out on an operational material after the patients treatment.
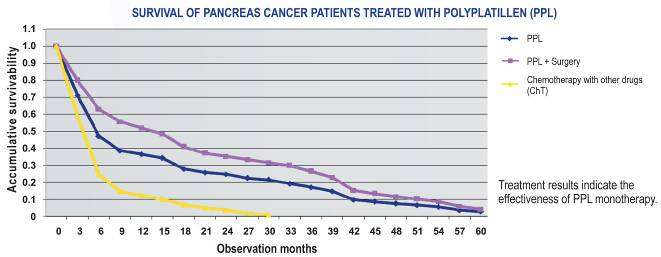
PPL application at treatment of the patients with PC
PC it is usually expressed in the advanced stages and is accompanied by a pain
syndrome, obstructive jaundice, cachexia. In this situation PPL was defined as a drug of
choice, considering its minimum toxicity.
Direct results of treatment of the patients were characterized by a tendency to the
improvement of an objective effect at the patients, in its turn, the 2-year survival rate is
observed only in the group of the patients treated with PPL that convincingly testifies to
a considerable PPL efficiency in a monochemotherapy mode at treatment of the
indicated pathology. The analysis of the survival rate of the patients with PC showed
that various morphological types of a tumor show identical sensitivity to PPL.
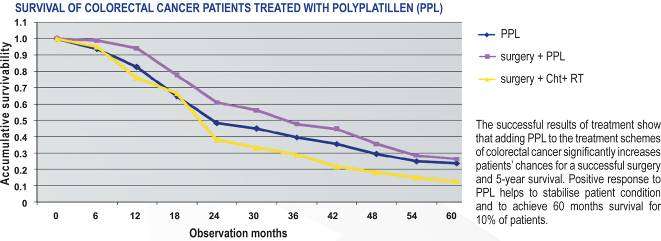
PPL application at treatment of the patients with CC
Treatment experience confirms that inclusion of chemotherapy in the regimens with
PPL into the program of treatment of the patients with CC considerably raises the
chances of successful surgery and increases 5-year survival rate.
Also at PPL application there are no expressed manifestations of toxicity at patients that
considerably facilitates a course of the disease and allows us to reach remission and
stabilization of the oncological process at advanced stages.
PPL prescription was accompanied by a positive reaction, besides the patients showed a
long stabilization of the disease, and the life expectancy at 10 % of the experimental
subjects made 60 months.
Subjective improvement of the general condition was observed: pain senses and
quantity of sanioserous excretions from a rectum decreased, however tumor immobility
remained at the former level.
At patients with locally generalized malignant process of a rectum after the second
course of the medication administration the improvement of the general condition that
showed itself in reduction and in due course in full termination of pain sensations and
sanioserous excretions is noticed, the passage of gastrointestinal contents also
improved.
The obtained data testifies to the advisability of PPL application for the patients with
advanced CC and also to the necessity of the further research of optimum ways of
administration of a highly effective drug.
Intraarterial chemotherapy at combination therapy of malignant neoplasms of
oropharynx
At intraarterial administration of PPL in chemotherapy regimens (which was
supplemented also with its administration in separate days of treatment regimen)
expressiveness of local and general toxic manifestations was considerably lower than
those developed at the patients receiving other antineoplastic medications.
Groups of the patients
Survival rate, %
Observation period
Quantity
from 3 to 5 years
from 5 and more
Application of the medicinal agent PPL in a system mode and also at intraarterial
administration caused the best direct results that were shown in greater frequency of full
and partial regression of a tumor and also cases of the process stabilization.
Groups: group 1 (only beam therapy); group 2А (polychemotherapy with cisplatin +
beam therapy); group 2B (polychemotherapy with PPL + beam therapy); group 3А
(intraarterial polychemotherapy with cisplatin + beam therapy); group 3B (intraarterial
polychemotherapy with PPL + beam therapy).
The results of treatment of malignant neoplasms depended on three basic indicators:
timely diagnostics, adequate treatment, follow-up and observation of the patients during
treatment and after it.
Application of intraarterial regional chemotherapy simultaneously with beam therapy at
treatment of the patients with malignant tumors of oropharynx allows us to achieve
optimum medical effect. Long regional intraarterial chemotherapy is the most
comprehensible way of delivery of the medical substances to a neoplasm.
Application of the modern national medication of PPL in the polychemotherapy
regimen in a combined mode or for intraarterial administration gives the best direct and
remote results at moderate side effects.
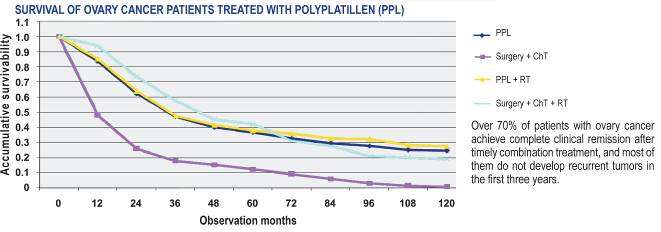
Character of treatment with PPL of the patients with ovarian carcinoma
At PPL application as neoadjuvant chemotherapy at ovarian carcinoma there is a
possibility to reach a low profile of toxicity. As the experience shows, inclusion of PPL
treatment into the regimens optimizes the results of treatment of the patients.
More than 70 % of the patients with malignant ovarian carcinoma reach full clinical
remission after the timely combined treatment, the majority of them do not develop
regression within the first 3 years. Treatment with PPL application in regimens is
recommended at regression.
4. Indications, contra-indications, mode of administration and doses
PPL it is applied for mono- or polychemotherapy at treatment of the patients with the
advanced forms of malignant ovarian carcinoma, liver, stomach, pancreas, large
intestine, lung, brain, head and neck tumors, sarcoma of bones and soft tissues, prostatic
gland, kidneys, adrenal glands, bladder, thyroid gland, carcinomatoses of an abdominal
cavity with ascites, and other types of polyserositis.
The drug is effective at chemoresistant tumors which are not sensitive to the treatment
by the standard chemotherapeutic regimens.
Application PPL is not recommended in case of hypersensibility to platinum
medications and also at generalization of tumor process, at a terminal condition of the
patient, during pregnancy and breast feeding.
Mode of administration and doses
Single doses of the drug of 200-250 mg/m2 are recommended at intravenous and
intraarterial ways of administration. Mode of administration – 3-6 times, by drop
infusion, every 24 hours; time of infusions – 3 hours.
The course dose of the drug makes 750-1500 mg/m2.
The recommended single doses of the drug at administration in an abdominal cavity are
200-250 mg/m2. Mode of administration – 4 times, by drop infusion, every 48-72 hours,
depending on a patient's condition. Time of infusions at intra-abdominal administration
makes 4-4.5 hours.
The course dose – 1000 mg/m2.
At all modes of administration it is necessary to dilute the drug in the ratio 1:1 with a
normal saline solution of sodium chloride with addition of 4-8 mg of dexamethasone to
the dropper.
It is necessary to repeat the treatment course each 3 weeks.
5. Polychemotherapy regimens
Non-small-cell lung cancer:
Regimen 1
Polyplatillen 200 мг/m2 intravenously by drop infusion 1-6 day;
etoposide 120 мг/m2, intravenously by drop infusion on 1, 3, 5 days;
Regimen2
Polyplatillen 200 mg/m2 intravenously by drop infusion on 1-6 day;
gemcitabine 1250 mg/m2 1, 8 days.
Regimen3
Polyplatillen 200 mg/m2 on 1-6 day;
taxotere 75 mg/m2 1 day;
Regimen4
Polyplatillen 200 mg/m2 on 1-6 day;
paclitaxel 175 mg/m2 1 day
Stomach cancer:
Regimen1
Polyplatillen 200 mg/m2 intravenously by drop infusion on 1-6 day;
etoposide 100 mg/m2, intravenously by drop infusion on 4, 5 and 6 days;
doxorubicin 20 mg/m2 intravenously by drop infusion on 1, 6 day.
Regimen2
Polyplatillen 200 mg/m2 intravenously by drop infusion on 1-6 day;
capecitabine 2000 mg/m2 a day per os in 2 doses on 1-14 day.
Regimen3
Polyplatillen 200 mg/m2 intravenously by drop infusion on 1-6 day;
5-ftoruratsil 1000 mg/m2 a day, intravenously continuously 24-hour infusion from the
1st till 5th day every 28 days.
Colorectal cancer:
Regimen 1
Polyplatillen 200 mg/m2 intravenously by drop infusion on 1-6 day;
leucovorin 400 mg/m2 2-hour infusion 1 day, before 5-ftoruratsil;
5-ftoruratsil 400 mg/m2 intravenously bolus dosing 1 day, after 2400 mg/m2
intravenously 46-hour infusion;
Regimen 2
Polyplatillen 200 mg/m2 intravenously by drop infusion 1-6 day;
capecitabine 2000 mg/m2 a day per os in 2 doses on 1-14 days.
Primary liver cancer:
Regimen 1
Polyplatillen 200 mg/m2 intravenously by drop infusion on 1-6 day;
capecitabine 2000 mg/m2 a day per os in 2 doses on 1-14 days;
Regimen 2
Polyplatillen 200 mg/m2 intravenously by drop infusion on 1-6 day;
doxorubicin 40 mg/m2 intravenously by drop infusion 1 day;
5-ftoruratsil 600 mg/m2 intravenously on 1-4 day
Pancreatic cancer:
Regimen1
Polyplatillen 200 mg/m2 intravenously by drop infusion on 1-6 day;
gemcitabine 1000 mg/m2 on 1, 8 days.
Skin melanoma:
Regimen 1
Polyplatillen 200 mg/ m2 intravenously by drop infusion on 1-6 day;
dacarbazine 800 mg/m2 intravenously by drop infusion 1 day;
vinblastine 1.6 mg/m2 intravenously1-5 day.
Regimen 2
Polyplatillen 200 mg/m2 intravenously by drop infusion on 1-6 day;
temozolomide 200 mg/m2 per os on 1-5 day.
Breast cancer:
Regimen 1
Polyplatillen 200 mg/m2 on 1-6 day;
cyclophosphamide 600 mg/m2 on 1 day;
doxorubicin 60 mg/m2 on 1 day.
Regimen 2
Polyplatillen 200 mg/m2 on 1-6 day;
taxotere 75 mg/m2 1 day.
Regimen 3
Polyplatillen 200 mg/m2 on 1-6 day;
vinorelbine 25 mg/m2 on 1, 6 day.
Regimen 4
Polyplatillen 200 mg/m2 on 1-6 day;
capecitabine 2000 mg/m2 a day 2 doses on 1-14 days
Ovarian cancer:
Regimen 1
Polyplatillen 200 mg/m2 intraperitoneally every 48-72 hours 4-times administration.
Time of infusions 4-4.5 hours.
Regimen 2
Polyplatillen 200 mg/m2 on 1-6 day;
cyclophosphamide 1000 mg/m2 1 day.
Regimen 3
Polyplatillen 200 mg/m2 on 1-6 day;
paclitaxel 175 mg/m2 1 day.
Regimen 4
Polyplatillen 200 mg/m2 on 1-6 day;
irinotecan 350 mg/m2 1 day.
Cervical cancer:
Regimen 1
Polyplatillen 200 mg/m2 on 1-6 day;
doxorubicin 60 mg/m2 1st day;
bleomycin 15 mg/m2 1st day.
Regimen 2
Polyplatillen 200 mg/m2 on 1-6 day;
taxotere 75 mg/m2 1st day;
irinotecan 180 mg/m2 – 1st day.
Endometrial cancer:
Regimen 1
Polyplatillen 200 mg/m2 on 1-6 day;
doxorubicin 50 mg/m2 – 1st day;
cyclophosphamide 600 mg/m2 – 1st day.
Regimen 2
paclitaxel 175 mg/m2 – 1st day;
Polyplatillen 200 mg/m2 on 1-6 day.
Adrenal carcinoma:
Regimen 1
Polyplatillen 200 mg/ m2 on 1-6 day;
doxorubicin 50 mg/ m2;
cyclophosphamide 600 mg/ m2 – 1 day;
Regimen 2
Polyplatillen 200 mg/m2 on 1-6 day;
etoposide 100 mg/m2 – 1 day;
bleomycin 30 mg/m2 – 1 day.
Soft tissue sarcoma:
Regimen 1
Polyplatillen 200 mg/m2 on 1-6 day;
doxorubicin 50 mg/m2 – on 2, 3, 4 day;
vincristine 1.5 mg/m2 – on 5 day;
cyclophosphamide 600 mg/m2 – on 6 day.
Osteogenic sarcoma:
Regimen 1
Polyplatillen 200 mg/m2 on 1-6 day;
cyclophosphamide 600 mg/m2 –1 day;
doxorubicin 50 mg/m2 –1 day.
Testicular cancer:
Regimen 1
Polyplatillen 200 mg/m2 on 1-6 day;
etoposide 100 mg/m2 on 1-5 day;
bleomycin 30 mg – every day for 9-12 weeks.
Oesophageal cancer:
Regimen 1
Polyplatillen 200 mg/m2 on 1-6 day;
epirubicin 60 mg/m2 – 1 day;
5-fluorouracil – 600 mg/m2 on 1-6 day.
6. Conclusions
Due to treatment with PPL medication it was possible to reach the high quality of life of the patients with generalization of oncological process on the III-IV stage, to increase life expectancy, to reduce expressiveness of a pain syndrome, endotoxicosis symptoms. Absence of the expressed manifestations of hemato-, nephro-, mielo-, neurotoxicity was noted, that provided a possibility of carrying out a full-scale chemotherapeutic treatment of the patients to whom standard antineoplastic therapy was counter-indicative. Owing to high antineoplastic activity and low toxicity, PPL application at palliative treatment of the patients with LC, SC, PC considerably improved the life quality of the patients (by ECOG scale) at the expense of reduction of expressiveness of the symptoms connected both with tumor process and influence of antineoplastic therapy. Application of regimens with PPL inclusion did not demand any supporting therapies and hyperhydration methods during treatment which are obligatory when using another medications of platinum. Indicators of the survival rate of the patients with LC, SC, PC of the IV stage treated with PPL allow us to diverge from a stereotype of treating such patients as hopeless from the point of view of possibilities of increase in life expectancy. PPL is a modern effective antineoplastic platiniferous medication the evidence to which are the results of the carried out experimental and clinical research. The modes and ways of PPL administration are developed at treatment of the patients with the advanced forms of LC, SC and PC and are recommended to be included in clinical protocols of the specialized help to the patients with malignant neoplasms.
7. List of the reference literature
1. Synopsis of a PhD thesis in Medical Sciences "Antineoplastic properties of Polyplatillen and efficiency of its application at treatment of the patients with the advanced forms of a stomach, pancreas and lung cancer". 2. Adapted clinical guidelines of the European Society of Medical Oncology and clinical protocols of specialized help to the patients with malignant neoplasms / Authors: Shalimov S.A., Ganul V.L., Lischishina E.M. and others // Kiev, 2007. – 187 p. 3. Vorobyov A.N., Shmykova E.V., Timoshev N.P., Vorobyov N.A. Regional intraarterial chemotherapy in complex treatment of patients with malignant neoplasms of oropharynx// Zaporozhye medical journal.– Volume 11, № 3.– 2009.– P. 11-17. 4. Shalimov S.A., Volchenskova I.I., Maydanevitch N.M., Maydanevitch Nat.M., Volobuyev M.A., Lenok O.V., Nezhin M.V. Application of Polyplatillen in treatment of the patients with malignant neoplasms of head and neck// Materials of the international medical pharmaceutical congress "Medications and life" February 21-24, 2006, Kiev.– P. 94. 5. Shalimov S.A., Volchenskova I.I., Glavatskiy A.Y., Semenova B.N., Hmelnitskiy G.V., Maydanevitch N.M., Stayn L.P., Nezhin M.V., Lenok O.V. Antineoplastic activity of Polyplatillen at cerebral tumours of a man // Materials of the international medical pharmaceutical congress "Medications and life" February 21-24, 2006, Kiev.– P. 95. 6. Shalimov S.A., Volchenskova I.I., Maydanevitch N.M., Maydanevitch Nat.M., Lenok O.V., Nezhin M.V. Change of possibilities of treatment of non-small-cell lung cancer with introduction into practice of a new platinum derivative – Polyplatillen // Materials of the international medical pharmaceutical congress "Medications and life" February 21-24, 2006, Kiev.– P. 118. 7. Shalimov S.A., Gladkiy A.V., Volchenskova I.I., Valetskiy V.L., Maydanevitch N.M., Zagorujko A.D. Optimum regimens of Polyplatillen application at treatment of malignant neoplasms of gastroenteric tract // International medical pharmaceutical congress "Medications and life" February 21-24, 2006, Kiev.– P. 96. 8. Shalimov S.A., Litvinenko A.A., Volchenskova I.I., Maydanevitch N.M. Polyplatillen in monochemotherapy at primary and metastatic liver cancer // Materials of the international medical pharmaceutical congress "Medications and life" February 21-24, 2006, Kiev.– P. 119. 9. Yatsenko L.D. Chemotherapy of inoperable tumors of intrathoracic organs and abdominal cavity organs // Oncology – Volume 9, № 1. – 2007. – P. 75-84. 10. Yatsenko L.D., Lyalkin S.A., Maydanevitch N.M. Application of Polyplatillen in chemotherapy of the advanced forms of pancreatic cancer // Clinical surgery. – 2008. – № 7. – P. 20-25.
Advantages of the active substance Polyplatillen in relation to platinum-based
medications with the active agents cisplatin and oxaliplatin
Indicators
Active agent Cisplatin
Active agent
Active agent
Oxaliplatin
Effect on the
No expressed toxicity,
organism
aggressive systemic
selective effect on the
lesion due to the
presence of platinum ions on DNA carrier
Supporting therapy
Not necessary. Is recommended for administration both in monoblock and in combined therapy
Life quality
Indications
Ovarian carcinoma (III
Adjuvant therapy of
Head and neck cancer,
large intestine cancer of liver, stomach, lungs,
III stage after full
kidneys, bowels,
epithelioma of head and primary tumor
ovarium, breasts,
neck, lung cancer,
resection; treatment of
prostatic gland,
urothelium cancer,
metastasizing colorectal bladder, skin
(melanoma) and bones,
brain cancer. Ascitic cancer types, carcinomatoses and polyserosites
A short instruction on medical application
Composition: active substance: Polyplatillen; 1 ml of concentrate contains 1.47 mg of Polyplatillen;
Additive agents: sodium chloride, sodium citrate, sodium hydroxide, fluorouracil (2,4-dioksi-5-
fluorinepyrimidine), water for injections.
Pharmaceutical dosage form. A concentrate for preparation of the solution for infusions
Pharmacotherapeutic group. Antineoplastic drugs. Platinum compounds. АТС code: L01XA.
Clinical performance.
Indications. Treatment of the patients with the advanced forms of a malignant tumor (ovarium, liver,
stomach, pancreas, large intestine, lung, tumors of brain and neck, sarcoma of bones and soft tissues),
including those accompanied by polyserositis with expressed cancerous toxemia, at carcinomatosis of
the abdominal cavity and ascites. The drug is applied at chemoresistant tumors tolerant to treatment by
generally accepted chemotherapeutic regimens. In a combination with others antineoplastic
medications Poliplatillen is applied to treat the patients with the advanced forms of a malignant tumor
(ovarium, alvus, liver, stomach, pancreas, large intestine, prostatic gland, kidneys, adrenal gland,
bladder, lung, tumors of brain, head and neck, oropharynx, thyroid body, sarcoma of bones and soft
tissues).
Contra-indications. Hypersensibility to platinum medications. Generalization of tumor process,
terminal condition of the patient, severe liver and kidneys function abnormality. Pregnancy and breast
feeding period.
Mode of administration and doses.
Single doses of the drug of 250-300 mg/m2 are recommended at intravenous and intraarterial ways of
administration. Mode of administration – 3-6 times, by drop infusion, every 24-48 hours; time of
infusions – 3-3.5 hours. The course dose of the drug makes 650-1300 mg/m2.
The recommended single doses of the drug at administration in an abdominal cavity are 150-300
mg/m2. Mode of administration – 3-6 times, by drop infusion, every 24-72 hours, depending on a
patient's condition. Time of infusions at intra-abdominal administration makes 4.5-5 hours. The course
dose – 650-1300 mg/m2.
Recommended dose of Polyplatillen under the regimens of the combined therapy makes Ѕ from the
calculated therapeutic dose.
At all modes of administration it is necessary to dilute the drug in the ratio 1:1 with a normal saline
solution of sodium chloride with addition of 4-8 mg of dexamethasone to the dropper. At
administration in an abdominal cavity it is recommended to add in a dropper 1 ml (5 000 units) of
heparine solution, 10-20 ml of 2 % lidocain solution.
The treatment course can be repeated in 3-4 weeks.
Application during pregnancy and breast feeding. The drug is not applied during pregnancy and
breast feeding. While being treated with Polyplatillen it is necessary to stop breast feeding.
Usage pattern. Polyplatillen should be applied by the doctors having experience in antineoplastic
chemotherapy.
Interaction with other medical agents and other kinds of interactions. In complex therapy
Polyplatillen can be used after methods and means which show mielodepressing and
immunosuppressive action. All necessary methods of immunotherapy should be used after carrying out
of a complete course of treatment with Polyplatillen.
Pharmacological properties. Polyplatillen is a high-molecular platinum compound with
deoxyribonucleic acid, shows antineoplastic properties due to its ability to block the synthesis of
nuclear DNA, permanently damages oncological cells which are in G1-S-phase of the cycle. Already
at the first administration of Poliplatillen its high antineoplastic activity and the tumor size reduction
may be noticed, thus there is no damage to the healthy tissues. The medication is effective at therapy
of tumors with the acquired medicinal resistance to other chemomedications.
Shelf life. 2 years.
Storage conditions: PPL should be stored in a dark place at temperature from15 to 25 C°. Sterility of
the depressurized drug in the concentrate is 7 days. It is not recommended to freeze the drug during its
storage time.
Source: http://www.polyplatillen.com.ua/files/Metod/Metod_recom.pdf
neurodegenerationresearch.eu
Updated daily at www.ResearchResearch.com 20 November 2014 Geoghegan-Quinn leaves mixed legacy – p13 ERA Less is more, or is it? – p5 Archiving Cologne collection's comeback after construction collapse – p6 Commission bids Glover a silent farewell Researchers call for clarification as CSA post is left to expire A tense relAtionship between the European by Laura Greenhalgh
Effects of losartan vs candesartan in reducing cardiovascular events in the primary treatment of hypertension
Journal of Human Hypertension (2010) 24, 263–273 & 2010 Macmillan Publishers Limited All rights reserved 0950-9240/10 $32.00 Effects of losartan vs candesartan inreducing cardiovascular events in theprimary treatment of hypertension SE Kjeldsen1, J Sta˚lhammar2, P Hasvold3, J Bodegard3, U Olsson4 and D Russell51Department of Cardiology, Oslo University Hospital, Ulleva˚l, Oslo, Norway; 2Department of Public Healthand Caring Sciences, Uppsala University, Uppsala, Sweden; 3Medical Department, AstraZeneca AS, Oslo,Norway; 4Statisticon AB, Uppsala, Sweden and 5Department of Neurology, Oslo University Hospital,Rikshospitalet, Oslo, Norway