Intlxhestjournal.chestpubs.org
Linezolid vs Vancomycin*Analysis of Two Double-Blind Studies of Patients
With Methicillin-Resistant Staphylococcus aureus
Nosocomial Pneumonia
Richard G. Wunderink, MD, FCCP; Jordi Rello, MD, PhD;Sue K. Cammarata, MD, FCCP; Rodney V. Croos-Dabrera, PhD; andMarin H. Kollef, MD, FCCP Objective: To assess the effect of baseline variables, including treatment, on outcome in patients
with nosocomial pneumonia due to methicillin-resistant Staphylococcus aureus (MRSA).
Design: Retrospective analysis of data from two prospective, randomized, double-blind studies.
Setting: Multinational study with 134 sites.
Patients: A total of 1,019 patients with suspected Gram-positive nosocomial pneumonia, including
339 patients with documented S aureus pneumonia (S aureus subset) and 160 patients with
documented MRSA pneumonia (MRSA subset).
Interventions: Linezolid, 600 mg, or vancomycin, 1 g, q12h for 7 to 21 days, each with aztreonam.
Measurements and results: Outcome was measured by survival and clinical cure rates (assessed 12
to 28 days after the end of therapy). Logistic regression analysis was used to determine the effect
of treatment and other baseline variables on outcome. Kaplan-Meier survival rates for linezolid
vs vancomycin were 80.0% (60 of 75 patients) vs 63.5% (54 of 85 patients) for the MRSA subset
(p ⴝ 0.03). Logistic regression analysis confirmed that the survival difference favoring linezolid
remained significant after adjusting for baseline variables (odds ratio [OR], 2.2; 95% confidence
interval [CI], 1.0 to 4.8; p ⴝ 0.05). Other baseline variables associated with significantly higher
survival rates in MRSA pneumonia were serum creatinine levels less than or equal to two times
the upper limit of normal and absence of cardiac comorbidities. Clinical cure rates for linezolid
vs vancomycin (excluding indeterminate or missing outcomes) were 59.0% (36 of 61 patients) vs
35.5% (22 of 62 patients) for the MRSA subset (p < 0.01). Logistic regression analysis confirmed
that the difference favoring linezolid remained significant after adjusting for baseline variables
(OR, 3.3; 95% CI, 1.3 to 8.3; p ⴝ 0.01). Other baseline variables associated with significantly
higher clinical cure rates in MRSA pneumonia were single-lobe pneumonia, absence of ventila-
tor-associated pneumonia, and absence of oncologic and renal comorbidities.
Conclusions: In this retrospective analysis, initial therapy with linezolid was associated with
significantly better survival and clinical cure rates than was vancomycin in patients with
nosocomial pneumonia due to MRSA.
(CHEST 2003; 124:1789 –1797)
Key words: linezolid; methicillin resistance; nosocomial pneumonia; regression analysis; Staphylococcus aureus; vancomycin
Abbreviations: APACHE ⫽ acute physiology and chronic health evaluation; CI ⫽ confidence interval; ELF ⫽ epithelial
lining fluid; EOT ⫽ end of treatment; EPIC ⫽ European Prevalence of Infection in Intensive Care; ITT ⫽ intent to treat; MIC ⫽ minimal inhibitory concentration; MRSA ⫽ methicillin-resistant Staphylococcus aureus; OR ⫽ odds ratio; VAP ⫽ ventilator-associated pneumonia Pneumonia was the most common nosocomial frequently reported pathogens, but Gram-positive
infection among patients in combined medical- pathogens are being reported with increasing fre- surgical ICUs in the National Nosocomial Infections quency. Staphylococcus aureus was the most fre- Surveillance1; nosocomial pneumonia occurred in31% of patients. Similarly, pneumonia was the lead- For editorial comment see page 1632
ing cause of ICU-acquired infection in the EuropeanPrevalence of Infection in Intensive Care (EPIC) quently reported isolate, and accounted for 17% of Study2; the crude mortality rate for ICU-acquired the pathogens in patients with nosocomial pneumo- pneumonia was 31%, and the associated odds ratio nia in the National Nosocomial Infections Surveil- (OR) for death was 1.9.
lance1 and for 30% of pathogens in patients in the In the past, Gram-negative aerobes were the most EPIC Study,2 which included pneumonia and other CHEST / 124 / 5 / NOVEMBER, 2003 Downloaded From: http://publications.chestnet.org/ on 10/07/2016
types of ICU-acquired infections. Methicillin-resis- The design of the two studies was identical and is summarized tant S aureus (MRSA) is an increasingly common briefly in this article. Both studies were randomized, double cause of infections and accounted for 60% of S blind, multicenter, multinational, and comparator controlled.
Both were designed as registration studies according to guide- aureus isolates in the EPIC Study.2 lines for industry specified by the US Food and Drug Adminis- Vancomycin has been the standard and, until re- tration for the assessment of patients with nosocomial pneumo- cently, only option for the treatment of patients with nia.7 The studies included 134 investigator sites in North MRSA infections; however, only limited data on the America, Europe, Israel, South Africa, Australia, and Latin treatment of patients with MRSA nosocomial pneumo- America, and enrolled patients from October 13, 1998, to April28, 2000; 70 sites (52.2%) participated in both studies. Studies nia are available from large comparator-controlled were approved by the Institutional Review Board for each studies. Two double-blind, registration studies3,4 of investigator site, and informed consent was obtained from all patients with Gram-positive nosocomial pneumonia patients or their legally authorized representative.
have recently been completed in which patients wererandomly assigned to receive initial empiric treatment Patients in the Prospective Studies with linezolid or vancomycin, each with aztreonam.
Each registration study was powered for equivalence, Adult men and women with pneumonia acquired after 48 h in an inpatient facility were eligible for enrollment. Patients had to and there were no outcome differences between treat- have at least two of the following: cough; purulent sputum; ment groups. We were intrigued by subset analyses auscultatory findings of pneumonia; dyspnea, tachypnea, or that revealed a survival difference favoring linezolid hypoxemia; or isolation of a respiratory pathogen from respiratory when patients were stratified by APACHE (acute or blood cultures. Patients also had to have at least two of the physiology and chronic health evaluation) II scores.5,6 following: fever or hypothermia, respiratory rate ⬎ 30 breaths/min, systolic BP ⬍ 90 mm Hg, pulse rate ⱖ 120 beats/min, The identical design of these studies and their com- altered mental status, need for mechanical ventilation, total bined sample size offer an opportunity to evaluate a peripheral WBC count ⬎ 10,000/L or ⬍ 4,500/L, or ⬎ 15% large database of patients with nosocomial pneumonia, immature neutrophils. Patients had to have radiographic findings including patients with S aureus and MRSA pneumo- of pneumonia (new or progressive infiltrates, consolidation, or nia. To assess the effect of baseline variables, including pleural effusion), adequate respiratory and sputum specimens forGram stain and culture, and life expectancy ⱖ 7 days. Exclusion treatment, on survival and clinical cure in patients with criteria were infecting Gram-positive organism resistant to either nosocomial pneumonia due to MRSA, we conducted a study medication; known or suspected meningitis, endocarditis, retrospective logistic regression analysis of data that osteomyelitis, or pulmonary disease that could preclude evalua- were collected prospectively in these studies.3,4 tion of therapeutic response (eg, granulomatous diseases, lungcancer, or another malignancy metastatic to the lung); history orevidence of coagulopathy; cystic fibrosis or suspected activetuberculosis; pheochromocytoma, untreated hyperthyroidism, Materials and Methods untreated or uncontrolled hypertension, or carcinoid syndrome;CD4 cell count ⬍ 200/L secondary to HIV infection; unstable Data from two prospective, randomized, double-blind, regis- psychiatric condition or seizure disorder requiring long-term tration studies3,4 comparing linezolid with vancomycin, each with medications; previous antibiotic treatment for ⬎ 24 h, unless aztreonam, in patients with suspected nosocomial pneumonia documented treatment failure or pathogen resistant to previous were combined and retrospectively analyzed to identify variables nonstudy antibiotic therapy; hypersensitivity to any study medi- that affected outcome as measured by survival and clinical cure cation; liver disease and total bilirubin more than five times the rates in patients with documented S aureus and MRSA pneu- upper limit of normal; and severe neutropenia (⬍ 500/L).
Patients were also excluded if they were pregnant, lactating, orunable to take adequate contraceptive measures.
*From Methodist Healthcare Memphis and the University ofTennessee (Dr. Wunderink), Memphis, TN; Joan XXIII Univer-sity Hospital (Dr. Rello), University Rovira i Virgili, Tarragona, Interventions and Assessments in the Prospective Studies Spain; Pharmacia (Drs. Cammarata and Croos-Dabrera),Kalamazoo, MI; and Department of Internal Medicine, Pulmo- Patients were randomly assigned to receive either linezolid, nary and Critical Care Division (Dr. Kollef), Washington Univer- 600 mg, or vancomycin, 1 g, which were administered by IV sity School of Medicine, St. Louis, MO.
infusion q12h for 7 to 21 consecutive days. Vancomycin dosage Dr. Wunderink is a consultant for, and has received research adjustments were required for patients with renal impairment support from Pharmacia. Drs. Wunderink and Rello are on the and were permitted for other patients according to the local speaker's bureau for Pharmacia. Drs. Cammarata and Croos-Dabrera are employees of Pharmacia. Dr. Kollef has received standard of care. To maintain blinding, a research pharmacist or honoraria from Pharmacia for lectures at national conferences.
equivalent nonstudy personnel monitored vancomycin dosages.
This study was supported by a grant from Pharmacia Corpora- All patients received concurrent aztreonam, 1 to 2 g q8h, for tion, Peapack, NJ.
possible mixed infection; aztreonam therapy could be discontin- Manuscript received January 21, 2003; revision accepted May 6, ued if no Gram-negative pathogens were identified. If no Gram- positive pathogens were identified, then the patient was dropped Reproduction of this article is prohibited without written permis- from the study.
sion from the American College of Chest Physicians (e-mail: Baseline microbiologic specimens were obtained for diagnosis through the day after enrollment. Acceptable culture methods Correspondence to: Richard G. Wunderink, MD, FCCP, MethodistHealthcare Memphis, 1265 Union Ave, Suite 501 Crews, Memphis, included expectorated sputum, endotracheal suction specimen, TN 38104-3499; e-mail: [email protected] and blood cultures as well as "invasive methods" such as pro- Clinical Investigations Downloaded From: http://publications.chestnet.org/ on 10/07/2016
tected specimen brush, BAL, transtracheal aspirate, transthoracic received at least one dose of either linezolid or aspirate, and thoracentesis. Final pathogen identification and vancomycin, and composed the ITT group (Fig 1). A susceptibility testing were determined at a central laboratory bymicrodilution techniques according to National Committee for total of 339 patients had documented S aureus Clinical Laboratory Standards guidelines.
pneumonia (S aureus subset), including 223 patients Survival analyses were conducted for all treated patients with (66%) in whom it was diagnosed by invasive pro- nosocomial pneumonia, and for the subsets with S aureus and cedure (ie, as protected specimen brush, BAL, MRSA pneumonia. For analysis of cure rates, patients were transtracheal or transthoracic aspiration, or thora- required to have had at least 5 days of therapy to be assessed ascured and at least 2 days of therapy to be assessed as failed.
centesis) or blood culture. All but one of the S aureus Clinical cure or failure was assessed at the end of treatment isolates had vancomycin minimal inhibitory concen- (EOT) and was repeated at the follow-up visit 12 to 28 days after trations (MICs) of ⱕ 2 g/mL, and 90% had MICs EOT. Results at the follow-up visit were used for all clinical of ⱕ 1 g/mL. A total of 160 had documented analyses. Clinical cure was defined as the resolution of baseline MRSA pneumonia (MRSA subset), including 95 signs and symptoms of pneumonia, with improvement or lack ofprogression of radiographic findings. Clinical failure was defined patients (59.4%) in whom it was diagnosed by inva- as persistence or progression of pneumonia, or the administration sive procedures or blood culture.
of a nonstudy antibiotic for pneumonia.
Patient characteristics were similar between the Patients whose follow-up outcomes were missing or indeter- two studies, and data were combined. Patient char- minate were excluded from analyses of cure rates (but not from acteristics for the S aureus and MRSA subsets are survival analyses). A follow-up outcome of missing or indetermi-nate was possible in the following scenarios. Patients who shown in Table 1. Characteristics for patients in- received ⬍ 2 days of treatment were assigned a follow-up cluded in the analyses of clinical cure (excluding outcome of missing. Patients assessed by the investigator as cured those with indeterminate or missing outcomes) were or improved at EOT, and whose assessment at follow-up was comparable to those for the corresponding ITT indeterminate (or not reported) were assigned an outcome of populations (data not shown).
indeterminate. Patients with an investigator's assessment ofclinical failure at EOT, followed by indeterminate (or notreported) at follow-up were assigned an outcome of failure.
Survival Analysis Patients assessed by the investigator as indeterminate at bothEOT and follow-up were also assigned an outcome of failure.
All patients were included in the ITT analysis of survival. Overall Kaplan-Meier survival rates for all Statistics in the Retrospective Analysis patients with nosocomial pneumonia (ITT group)were 80.9% (424 of 524 patients) for linezolid and All results were locked into the database before the retrospec- 77.8% (385 of 495 patients) for vancomycin tive analysis was conducted. Statistics were calculated using (p ⫽ 0.21). As shown in Figure 2, Kaplan-Meier Statistical Analysis System Version 6.12 (SAS Institute; Cary,NC). The Kaplan-Meier method was used to assess survival rate.
survival rates for linezolid vs vancomycin therapy 2 test was used to assess the association between treatment and were 78.0% (131 of 168 patients) vs 70.8% (121 of categorical variables. Stepwise analysis was performed using 171 patients) for the S aureus subset (p ⫽ 0.13), and logistic regression to identify the most parsimonious model for 80.0% (60 of 75 patients) vs 63.5% (54 of 85 patients) clinical cure and survival. Baseline variables used as potential for the MRSA subset (p ⫽ 0.03). Similar trends were predictors in the stepwise analysis were similar to those used inanother logistic regression analysis8 and included treatment with seen in the 223 patients in whom the presence of S linezolid or vancomycin; age ⬍ or ⱖ 65 years; APACHE II score aureus was confirmed at baseline by invasive diag- ⱕ 20 or ⬎ 20; single- or multiple-lobe pneumonia; presence or nostic procedure or blood culture; 79% (86 of 109 absence of pleural effusion, bacteremia, and ventilator-associated patients) receiving linezolid and 72% (82 of 114 pneumonia (VAP); bilirubin ⱕ or ⬎ 41.0 mol/L (2.4 mg/dL); patients) receiving vancomycin survived (p ⫽ 0.23).
creatinine ⱕ or ⬎ 229.8 mol/L (2.6 mg/dL) for men and ⱕ or ⬎ 212.2 mol/L (2.4 mg/dL) for women; and presence or In the subset with MRSA confirmed by invasive absence of cardiac, diabetic, hepatic, oncologic, renal, respira- procedure or blood culture, 85% (34 of 40 patients) tory, or vascular comorbidities. Stepwise analyses used signifi- receiving linezolid and 67% (37 of 55 patients) cance levels of 0.25 for entry in the model and 0.10 for staying in receiving vancomycin survived (p ⫽ 0.05).
the model; statistical significance was assessed by the likelihood Bacteremia was confirmed in 13% (44 of 339 ratio test. ORs, 95% confidence intervals (CIs), and p values forbaseline variables associated with clinical cure and survival were patients) from whom S aureus was isolated, includ- calculated for the most parsimonious logistic regression model; ing 6% (22 of 339 patients) with MRSA bacteremia.
p ⱕ 0.05 was considered statistically significant.
Of the patients with S aureus bacteremia, 18 of 22linezolid-treated patients and 16 of 22 vancomycin-treated patients survived (p ⫽ 0.47). Of the patients with MRSA bacteremia, 7 of 8 linezolid-treatedpatients and 9 of 14 vancomycin-treated patients survived (p ⫽ 0.24).
A total of 1,019 patients with suspected nosoco- Significant predictors of survival in all patients mial pneumonia were enrolled in the two studies,3,4 with nosocomial pneumonia were linezolid therapy CHEST / 124 / 5 / NOVEMBER, 2003 Downloaded From: http://publications.chestnet.org/ on 10/07/2016
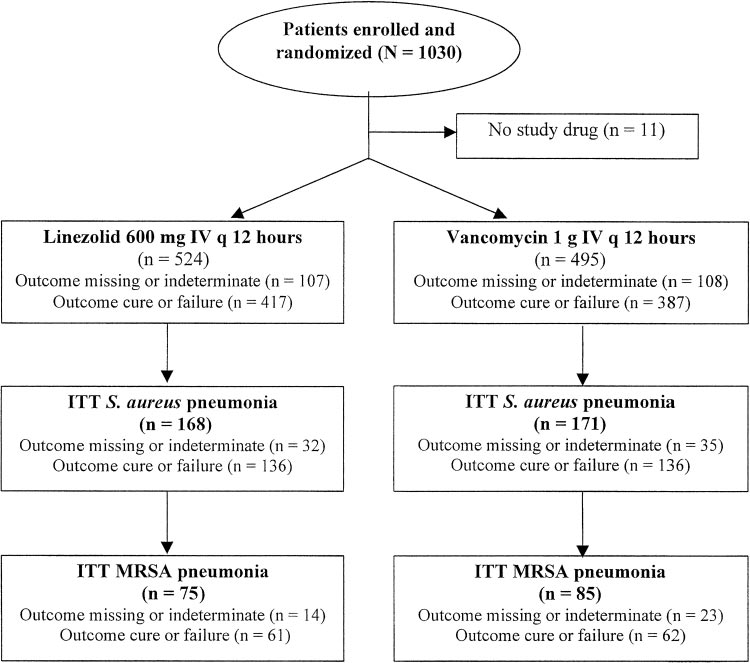
Figure 1. Flow diagram for patients with nosocomial pneumonia.
(OR, 1.4; 95% CI, 1.0 to 2.0; p ⫽ 0.03), APACHE II
in 37 linezolid recipients and 42 vancomycin recipi-
score ⱕ 20 (OR, 2.5; 95% CI, 1.7 to 3.7; p ⬍ 0.01),
ents for the following reasons: death (n ⫽ 9 and
single-lobe pneumonia (OR,1.9; 95% CI, 1.3 to 2.6;
n ⫽ 12), loss to follow-up and other administrative
p ⬍ 0.01), age ⬍ 65 years (OR, 2.3; 95% CI, 1.6 to
reasons (n ⫽ 11 and n ⫽ 16), isolation of Gram-
3.3; p ⬍ 0.01), and serum creatinine less than or
negative pathogens only (n ⫽ 12 and n ⫽ 10), and
equal to two times the upper limit of normal (OR,
adverse events (n ⫽ 5 and n ⫽ 4), respectively. Clin-
2.6; 95% CI, 1.4 to 4.9; p ⬍ 0.01). As shown in Table
ical outcome was indeterminate at follow-up in 70
2, significant predictors of survival in the S aureus
linezolid and 66 vancomycin recipients; these pa-
subset were APACHE II score ⱕ 20, and absence of
tients were assessed as cured or improved at their
cardiac and renal comorbidities. Logistic regression
analysis confirmed that the survival difference favor-
In patients who had a clinical outcome assessment
ing linezolid therapy in the MRSA subset remained
of cure or failure, overall clinical cure rates for all
significant after adjusting for baseline variables. Ad-
patients with nosocomial pneumonia were 53.0%
ditional significant predictors of survival in the
(221 of 417 patients) for linezolid and 52.2% (202 of
MRSA subset were serum creatinine less than orequal to two times the upper limit of normal and
387 patients) for vancomycin (p ⫽ 0.82). As shown in
absence of cardiac comorbidities.
Figure 3, clinical cure rates for linezolid vs vanco-mycin therapy were 51.5% (70 of 136 patients) vs43.4% (59 of 136 patients) for the S aureus subset
Clinical Cure Analysis
(p ⫽ 0.18), and 59.0% (36 of 61 patients) vs 35.5%
In the clinical cure regression analysis, 804 of
(22 of 62 patients) for the MRSA subset (p ⬍ 0.01).
1,019 treated patients were included and 215 were
Similar trends were seen in patients in whom the
excluded because their clinical outcome at follow-up
presence of S aureus was confirmed by invasive
was either missing (n ⫽ 79) or indeterminate
diagnostic procedure or blood culture; 51% (47 of 92
(n ⫽ 136). Clinical outcome was missing at follow-up
patients) receiving linezolid and 43% (39 of 90
Clinical Investigations
Downloaded From: http://publications.chestnet.org/ on 10/07/2016
Table 1—Patient Characteristics, Including Those Used in Logistic Regression Analysis*
ITT S aureus (n ⫽ 339)
ITT MRSA (n ⫽ 160)
Linezolid (n ⫽ 168)
Vancomycin (n ⫽ 171)
Linezolid (n ⫽ 75)
Vancomycin (n ⫽ 85)
Treatment duration†
APACHE II score ⬎ 20
Chest radiographic variables
Multilobe pneumonia
Bilirubin ⬎ 41.0 mol/L (2.4 mg/dL)
Serum creatinine ⬎ 229.8 mol/L‡
*Data are presented as No. of patients (%) unless otherwise indicated.
†Characteristic not included in logistic regression analysis.
‡Less than 229.8 mol/L (2.6 mg/dL) for men and 212.2 mol/L (2.4 mg/dL) for women.
patients) receiving vancomycin had a clinical cure
logic and renal comorbidities. Additional significant
(p ⫽ 0.30). In the subset with MRSA confirmed by
predictors of cure in the S aureus subset were
invasive procedure or blood culture, 58% (19 of 33
APACHE II score ⱕ 20 and absence of cardiac
patients) receiving linezolid and 33% (13 of 39
comorbidities. Logistic regression analysis confirmed
patients) receiving vancomycin had a clinical cure
that the difference in clinical cure rate favoring
(p ⫽ 0.04).
linezolid therapy in the MRSA subset remained
Of the patients with S aureus bacteremia, 10 of 18
significant after adjusting for baseline variables.
linezolid-treated patients and 7 of 16 vancomycin-treated patients had a clinical cure (p ⫽ 0.49). Of thepatients with MRSA bacteremia, four of six linezolid-
treated patients and three of eight vancomycin-treated patients had a clinical cure (p ⫽ 0.28).
As seen in other analyses,9–14 our retrospective
Significant predictors of clinical cure in all patients
analysis identified the presence of some baseline
with nosocomial pneumonia were APACHE II score
variables, such as APACHE II score ⱕ 20 or absence
ⱕ 20 (OR, 2.9; 95% CI, 1.9 to 4.7; p ⬍ 0.01),
of comorbidities, as independent predictors of sur-
single-lobe pneumonia (OR, 1.7; 95% CI, 1.3 to 2.4;
vival. However, the only baseline variable amenable
p ⬍ 0.01), absence of VAP (OR, 2.1; 95% CI, 1.5 to
to intervention in this setting is the choice of initial
2.9; p ⬍ 0.01), and absence of oncologic (OR, 2.3;
antimicrobial therapy. The importance of appropri-
95% CI, 1.3 to 4.0; p ⬍ 0.01) and renal comorbidities
ate initial empiric therapy is well known. Crude
(OR, 2.3; 95% CI, 1.4 to 3.8; p ⬍ 0.01). As shown in
mortality rates in critically ill patients are 8.5 to
Table 3, significant predictors of clinical cure in both
39.9% lower if initial empiric antimicrobial therapy is
the S aureus and MRSA subsets were single-lobe
appropriate than if modification is required.15–17
pneumonia, absence of VAP, and absence of onco-
Whereas appropriate therapy is necessary, ours is the
CHEST / 124 / 5 / NOVEMBER, 2003
Downloaded From: http://publications.chestnet.org/ on 10/07/2016
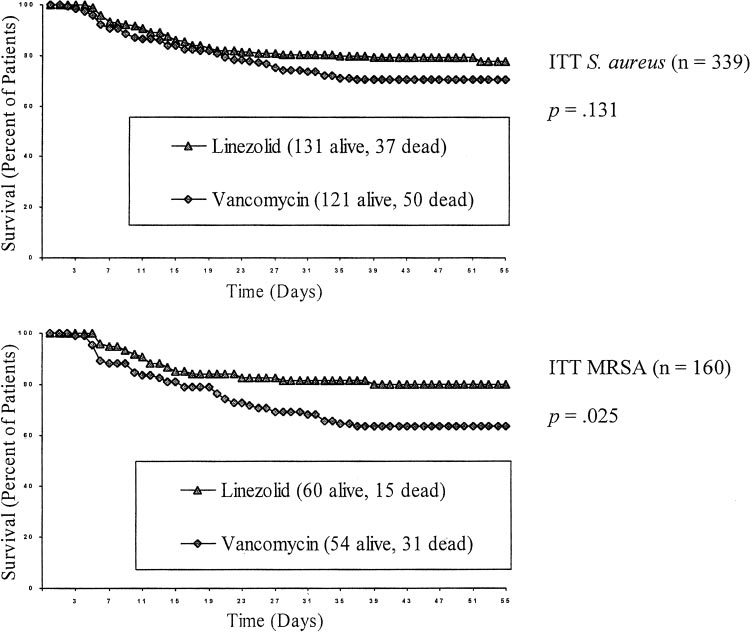
Figure 2. Kaplan-Meier survival curves for uncensored data.
first analysis, based on randomized, double-blind
(80.0% vs 63.5%, p ⫽ 0.03) and clinical cure rates
clinical study data,3,4 to demonstrate a survival ad-
(59.0% vs 35.5%, p ⬍ 0.01) if they were treated with
vantage for one appropriate antimicrobial agent over
linezolid than with vancomycin. Patients were en-
another appropriate agent in patients treated for
rolled based on their clinical diagnoses, before cul-
MRSA pneumonia.
ture results were known; a potential exists for imbal-
Patients in the MRSA subset had better survival
ances to occur between treatment groups in riskfactors that might have affected outcomes. However,logistic regression analysis confirmed that the advan-
Table 2—Results of Logistic Regression Analysis for
tages favoring linezolid therapy remained significant
Survival in Patients With Nosocomial Pneumonia
after adjusting for differences in baseline variables in
the subset with MRSA pneumonia.
Only two other randomized studies8,18 of patients
ITT S aureus (n ⫽ 339)
Linezolid therapy
with Gram-positive nosocomial pneumonia in which
vancomycin was the control agent are available.
APACHE II score ⱕ 20
Quinupristin/dalfopristin and vancomycin had equiv-
Single-lobe pneumonia
alent clinical cure rates in all patients (43.3% vs
Presence of pleural effusion
45.3%; 95% CI, ⫺ 13.2 to 9.3; n ⫽ 298) and statis-
Absence of cardiac comorbidities
Absence of renal comorbidities
tically equivalent clinical cure rates in the subset
ITT MRSA (n ⫽ 160)
with MRSA pneumonia (19.4% vs 40.0%; 95% CI,
Linezolid therapy
⫺ 46.2 to 4.9; n ⫽ 51).8 Linezolid and vancomycin
APACHE II score ⱕ 20
had equivalent clinical cure rates in all patients with
Presence of pleural effusion
pneumonia (51.3% vs 50.0%, n ⫽ 71) and in the
Creatinine ⱕ 229.8 mol/L*
11.9 (1.1–125.0)
Absence of cardiac comorbidities
subset with MRSA pneumonia (52.2% vs 53.8%,n ⫽ 49)18; this study was not included in the current
*Less than or equal to 229.8 mol/L (2.6 mg/dL) for men
and ⱕ 212.2 mol/L (2.4 mg/dL) for women.
analysis because the protocol was different and
†Significant at 0.05 level.
allowed enrollment of patients who had other types
Clinical Investigations
Downloaded From: http://publications.chestnet.org/ on 10/07/2016
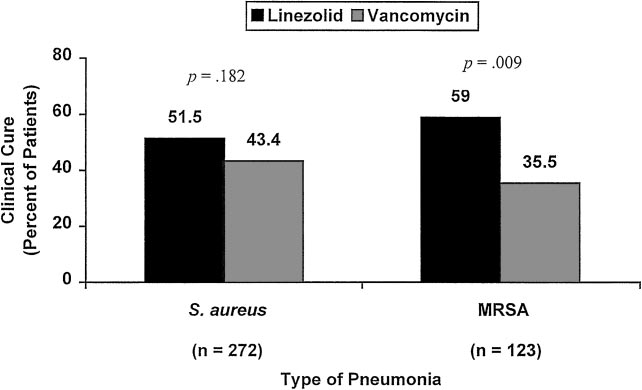
Figure 3. Clinical cure rates for linezolid and vancomycin therapy in patients with Gram-positive,nosocomial pneumonia. Data from patients with indeterminate or missing clinical outcomes wereexcluded.
of infections, such as skin and soft-tissue infections.
(9.6 mg/kg vs 40.6 mg/L) and at 12 h (2.8 mg/kg vs
Survival rates were not reported in either study.8,18
6.7 mg/L) in 30 patients.19 In contrast, mean con-
An important difference between those two studies
centrations of linezolid were higher in epithelial
and ours was the enrollment of more than three
lining fluid (ELF) than in plasma at 4 h (64.3 g/mL
times as many patients with nosocomial Gram-posi-
vs 7.3 g/mL) and at 12 h (24.3 g/mL vs 7.6
tive pneumonia and MRSA pneumonia in the com-
g/mL) in 25 volunteers,20 and in ELF than in blood
bined linezolid studies3,4 than in the next largest
at 2 to 4 h (29.5 g/mL vs 15.9 g/mL) and at 6 to
10 h (26.6 g/mL vs 10.9 g/mL) in 10 patients.21
One possible reason for the association between
The distribution of antimicrobial agents may be
linezolid and improved survival is the poor penetra-
different into ELF and lung tissue; however, the
tion of vancomycin into the lungs seen in pharmaco-
ratio of vancomycin concentration in the lung sample
kinetic studies. Mean concentrations of vancomycin
to that in serum or plasma was higher in the study
in lung tissue were lower than those in serum at 1 h
involving lung tissue19 than in an earlier study ofvancomycin concentrations in ELF.22 The collectiveresults of these studies indicate that linezolid, but
Table 3—Results of Logistic Regression Analysis for
not vancomycin, concentrations exceeded the MIC
Clinical Cure in Patients With Nosocomial Pneumonia*
breakpoint for susceptible S aureus throughout the
12-h dosing interval; the break point is 4 g/mL forboth antimicrobial agents.
S aureus pneumonia (n ⫽ 272)
Our study design had some limitations. Our study
Linezolid therapy
APACHE II score ⱕ 20
was a retrospective subgroup analysis. However, the
Single-lobe pneumonia
data were from prospective, randomized, double-
blind studies, and the database was locked before the
Absence of cardiac comorbidities
retrospective analysis was conducted. The predeter-
Absence of oncologic comorbidities
mined primary end point of both studies was clinical
Absence of renal comorbidities
13.5 (3.0–62.5)
MRSA pneumonia (n ⫽ 123)
cure, which was assessed at follow-up and defined
Linezolid therapy
conservatively; clinical outcome was assessed as fail-
Single-lobe pneumonia
ure if the assessment was either failure or indeter-
minate at EOT followed by indeterminate at follow-
Absence of oncologic comorbidities
21.7 (3.7–125.0) ⬍ 0.001†
up. Although not a prospectively defined end point,
Absence of renal comorbidities
16.4 (3.2–83.3)
Absence of hepatic comorbidities
mortality is an objective, clinically relevant parame-ter. In addition, our analysis included microbiologi-
*Data from patients with clinical outcomes assessed as indeterminate
or missing were excluded.
cally documented cases of S aureus nosocomial
†Significant at 0.05 level.
pneumonia from the entire ITT population. Sec-
CHEST / 124 / 5 / NOVEMBER, 2003
Downloaded From: http://publications.chestnet.org/ on 10/07/2016
ondly, results of two studies were combined; how-
pneumonia including VAP,15–17 appropriate empiric
ever, the protocols were identical, approximately half
treatment must be initiated promptly. The results of
of the investigators were identical, and we found no
this retrospective analysis suggest that initial empiric
differences between the two study populations.
therapy with linezolid should be considered in pa-
Combining studies allowed us to examine the largest
tients with suspected nosocomial pneumonia who
cohort of patients with MRSA pneumonia enrolled
are at risk for infection due to MRSA. Candidates for
in randomized, double-blind studies identified by a
this approach may include patients who are admitted
computerized search of the published literature,
to facilities where MRSA is present, whose stain
which in turn reduced the risk of  error and allowed
is positive for Gram-positive cocci, and who have risk
us to confirm findings noted in the original co-
factors for MRSA as shown epidemiologic studies.30,31
horts.5,6 In contrast, the lack of significant differencein clinical cure rates between vancomycin and quinu-
ACKNOWLEDGMENT: We thank M. Michele Wesley, Beth A.
Lesher, and Cindy W. Hamilton for assistance with manuscript
pristin/dalfopristin in the MRSA subset of the study
preparation; Mary Catherine Krug for programming assistance;
by Fagon and colleagues8 (40% vs 19.4%) may have
and Vu H. Le for statistical support.
been attributable to the small sample size.
The optimal method for dosing vancomycin has
been debated.23–26 The dose of vancomycin chosenfor the registration studies, 1 g q12h, is the approved
1 Richards MJ, Edwards JR, Culver DH, et al. Nosocomial
infections in combined medical-surgical intensive care units
dose, approximates the 15 mg/kg dose in a standard
in the United States. Infect Control Hosp Epidemiol 2000;
guide,27 and is identical to that used in other ran-
domized studies8,18 of vancomycin. Pharmacokinetic
2 Vincent JL, Bihari DJ, Suter PM, et al. The prevalence of
monitoring is often advocated to avoid toxicity or
nosocomial infection in intensive care units in Europe: results
even to improve efficacy, especially when combined
of the European Prevalence of Infection in Intensive Care(EPIC) Study; EPIC International Advisory Committee.
with pharmacodynamic modeling28,29; and our pro-
JAMA 1995; 274:639 – 644
tocol did allow dosage adjustments and pharmacoki-
3 Rubinstein E, Cammarata S, Oliphant T, et al. Linezolid
netic monitoring according to the local standard of
(PNU-100766) versus vancomycin in the treatment of hospi-
talized patients with nosocomial pneumonia: a randomized,
Finally, the use of quantitative cultures was not
double-blind, multicenter study. Clin Infect Dis 2001; 32:402– 412
required for diagnosis of nosocomial pneumonia,
4 Wunderink RG, Cammarata SK, Oliphant TH, et al. Lin-
either at study entry or on continuation. More than
ezolid versus vancomycin in the treatment of patients with
50% of the patients in both the S aureus and MRSA
nosocomial pneumonia: continuation of a randomized, dou-
subgroups had diagnoses made by invasive methods
ble-blind, multicenter study. Clin Ther 2003; 25:980 –992
or blood culture. The use of sputum or tracheal
5 Cammarata SK, Wunderink RG, Timm JA, et al. Efficacy of
linezolid in patients with nosocomial pneumonia based on
suctioning for culture reflects common medical prac-
severity of illness as determined by baseline APACHE score:
tice in the United States, where most critically ill
Interscience Conference on Antimicrobial Agents and Che-
patients continue to be treated according to the
motherapy 2000; 40:487; Poster 2234
results of nonquantitative, noninvasive diagnostic
6 Cammarata SK, Hempsall KA, Oliphant T. Linezolid in
studies. Interestingly, the response pattern, both for
nosocomial pneumonia: efficacy results by severity of illnessas determined by APACHE II scores [abstract] Chest 2001;
survival and clinical cure, in patients diagnosed by
120(4 Suppl):168S
invasive methods or blood culture mirrored the
7 U. S. Department of Health and Human Services, Food and
results in the entire cohort. Therefore, the results of
Drug Administration, Center for Drug Evaluation and Re-
our study are likely to represent the responses to
search (CDER). Guidance for industry: nosocomial pneumo-
antimicrobial therapy in patients with MRSA pneu-
nia; developing antimicrobial drugs for treatment, 1998.
Available at: http://www.fda.gov/cder/guidance/2571dft.pdf.
monia by usual nonquantitative diagnostic methods.
Accessed October 8, 2003
In conclusion, linezolid therapy was associated
8 Fagon J, Patrick H, Haas DW, et al. Treatment of Gram-
with significantly higher survival rates and clinical
positive nosocomial pneumonia: prospective randomized
cure rates than was vancomycin therapy in patients
comparison of quinupristin/dalfopristin versus vancomycin;
with nosocomial pneumonia due to MRSA. This
Nosocomial Pneumonia Group. Am J Respir Crit Care Med2000; 161:753–762
benefit remained significant after using logistic re-
9 Fagon JY, Chastre J, Domart Y, et al. Mortality due to
gression analysis to adjust for baseline variables.
ventilator-associated pneumonia or colonization with Pseudo-
Future studies may document the benefit of this
monas or Acinetobacter species: assessment by quantitative
approach, but fully powered, comparator-controlled,
culture of samples obtained by a protected specimen brush.
prospective studies in patients with MRSA nosoco-
Clin Infect Dis 1996; 23:538 –542
10 Fine MJ, Smith MA, Carson CA, et al. Prognosis and
mial pneumonia would be difficult to complete.
outcomes of patients with community-acquired pneumonia: a
Because of the documented importance of initial
meta-analysis. JAMA 1996; 275:134 –141
treatment in critically ill patients with nosocomial
11 Ibrahim EH, Ward S, Sherman G, et al. A comparative
Clinical Investigations
Downloaded From: http://publications.chestnet.org/ on 10/07/2016
analysis of patients with early-onset vs late-onset nosocomial
entry into pulmonary lining fluid by bronchoalveolar lavage in
pneumonia in the ICU setting. Chest 2000; 117:1434 –1442
critically ill patients. Antimicrob Agents Chemother 1993;
12 Ibrahim EH, Tracy L, Hill C, et al. The occurrence of
ventilator-associated pneumonia in a community hospital: risk
23 Cantu TG, Yamanaka-Yuen NA, Lietman PS. Serum vanco-
factors and clinical outcomes. Chest 2001; 120:555–561
mycin concentrations: reappraisal of their clinical value. Clin
13 Sarnak MJ, Jaber BL. Pulmonary infectious mortality among
Infect Dis 1994; 18:533–543
patients with end-stage renal disease. Chest 2001; 120:1883–
24 Moellering RC Jr. Monitoring serum vancomycin levels:
climbing the mountain because it is there? Clin Infect Dis
14 Timsit JF, Chevret S, Valcke J, et al. Mortality of nosocomial
1994; 18:544 –546
pneumonia in ventilated patients: influence of diagnostic
25 Karam CM, McKinnon PS, Neuhauser MM, et al. Outcome
tools. Am J Respir Crit Care Med 1996; 154:116 –123
assessment of minimizing vancomycin monitoring and dosing
15 Kollef MH, Sherman G, Ward S, et al. Inadequate antimi-
adjustments. Pharmacotherapy 1999; 19:257–266
crobial treatment of infections: a risk factor for hospital
26 Tobin CM, Darville JM, Thomson AH, et al. Vancomycin
mortality among critically ill patients. Chest 1999; 115:462–
therapeutic drug monitoring: is there a consensus view? The
results of a UK National External Quality Assessment Scheme
16 Alvarez-Lerma F. Modification of empiric antibiotic treat-
(UK NEQAS) for Antibiotic Assays questionnaire. J Antimi-
ment in patients with pneumonia acquired in the intensive
crob Chemother 2002; 50:713–718
care unit: ICU-Acquired Pneumonia Study Group. Intensive
27 Gilbert DN, Moellering RC, Jr, Sande MA. The Sanford
Care Med 1996; 22:387–394
Guide to Antimicrobial Therapy. Hyde Park, VT: Antimicro-
17 Rello J, Gallego M, Mariscal D, et al. The value of routine
bial Therapy, 2001; 1–142
microbial investigation in ventilator-associated pneumonia.
28 Schentag JJ. Antimicrobial action and pharmacokinetics/phar-
Am J Respir Crit Care Med 1997; 156:196 –200
macodynamics: the use of AUIC to improve efficacy and
18 Stevens DL, Herr D, Lampiris H, et al. Linezolid versus
avoid resistance. J Chemother 1999; 11:426 – 439
vancomycin for the treatment of methicillin-resistant Staph-
29 Moise PA, Forrest A, Bhavnani SM, et al. Area under the
ylococcus aureus infections. Clin Infect Dis 2002; 34:1481–
inhibitory curve and a pneumonia scoring system for predict-
ing outcomes of vancomycin therapy for respiratory infections
19 Cruciani M, Gatti G, Lazzarini L, et al. Penetration of
by Staphylococcus aureus. Am J Health Syst Pharm 2000;
vancomycin into human lung tissue. J Antimicrob Chemother
57(Suppl 2):S4 –S9
1996; 38:865– 869
30 Rello J, Torres A, Ricart M, et al. Ventilator-associated
20 Conte JE Jr., Golden JA, Kipps J, et al. Intrapulmonary
pneumonia by Staphylococcus aureus: comparison of methi-
pharmacokinetics of linezolid. Antimicrob Agents Chemother
cillin-resistant and methicillin-sensitive episodes. Am J Respir
2002; 46:1475–1480
Crit Care Med 1994; 150:1545–1549
21 Honeybourne D, Tobin C, Jevons G, et al. Intrapulmonary
31 Pujol M, Corbella X, Pena C, et al. Clinical and epidemio-
penetration of linezolid [abstract]. Chest 2002; 122(4 Suppl):
logical findings in mechanically-ventilated patients with
methicillin-resistant Staphylococcus aureus pneumonia. Eur
22 Lamer C, de Beco V, Soler P, et al. Analysis of vancomycin
J Clin Microbiol Infect Dis 1998; 17:622– 628
CHEST / 124 / 5 / NOVEMBER, 2003
Downloaded From: http://publications.chestnet.org/ on 10/07/2016
Source: http://intlxhestjournal.chestpubs.org/data/Journals/CHEST/22000/1789.pdf
The ways for development of environmentally safe solid composite propellants
Progress in Propulsion Physics 1 (2009) 63-80 DOI: 10.1051/eucass/200901063 © Owned by the authors, published by EDP Sciences, 2009 THE WAYS FOR DEVELOPMENT OF ENVIRONMENTALLY SAFE SOLID COMPOSITE PROPELLANTS D. B. Lempert, G. B. Manelis, and G. N. Nechiporenko The paper considers a wide set of issues concerning the creation of highenergetic solid composite propellants causing the minimal polluting e¨ecton the environment. Thereby, the level of toxicity of products of di¨er-ent compositions is discussed and propellants with perchlorates oxidizersare compared with propellants with halogen free oxidizers (mainly, am-monium dinitramide, HMX, CL-20). The main methods for creatingcompositions having a required performance and the highest energeticcharacteristics are also under discussion. The dependences of the spe-ci¦c impulse on the mode of formulation arrangement and on the com-pounds£ properties (i.e., formation enthalpy, density, element content)are demonstrated. The main principles for the maximal use of ener-getic potential of chemical substances are under consideration. Theseare the proper selection of the binder type which would be optimal forthe given mixture of oxidizer with fuel (or energetic) component and theopportunity of using metals and their hydrides (mainly, aluminum hy-dride (AH)). Main obstacles in using di¨erent kinds of compositions, aswell as advantages of speci¦c propellants are under consideration as well.A special attention is paid to the interrelationship between the energeticparameter and other performances (thermal stability, combustion law,sensitivity, and compatibility).
web.mit.edu
Real Time and Noninvasive Monitoring of Dry Powder Blend Homogeneity Chee-Kong Lai, David Holt, James C. Leung, and Charles L. Cooney Dept. of Chemical Engineering, Massachusetts Institute of Technology, Cambridge, MA 02139 Gokaraju K. Raju Sloan School of Management, MIT, Cambridge, MA 02139 Peter Hansen Union Biometrica, Inc., 19 Ward Street, Somerville, MA 02143