Microsoft word - dear author.doc
THE JOURNAL OF PHARMACOLOGY AND EXPERIMENTAL THERAPEUTICS J Pharmacol Exp Ther 351:1–7, November 2014 Copyright ª 2014 by The American Society for Pharmacology and Experimental Therapeutics Attenuated Aortic Vasodilation and Sympathetic Prejunctional Facilitation in Epinephrine-Deficient Mice: Q:1 Selective Impairment of b2-Adrenoceptor Responses Mónica Moreira-Rodrigues, Ana L. Graça, Marlene Ferreira, Joana Afonso, Paula Serrão,Manuela Morato, Fátima Ferreirinha, Paulo Correia-de-Sá, Steven N. Ebert,and Daniel MouraLaboratory of General Physiology (M.M.-R.) and Laboratory of Pharmacology and Neurobiology (F.F., P.C.), Unit forMultidisciplinary Investigation in Biomedicine, Institute of Biomedical Sciences Abel Salazar, University of Porto; Department ofPharmacology and Therapeutics, Faculty of Medicine, University of Porto (A.L.G., M.F., J.A., P.S., D.M.); Neuropharmacology,Institute of Molecular and Cellular Biology, University of Porto (M.M., D.M.); Center for Drug Discovery and Innovative Medicines,University of Porto, Porto, Portugal (M.M.-R., A.L.G., M.F., J.A., P.S., M.M., F.F., P.C., D.M.); Laboratory of Pharmacology,Department of Drug Sciences, Faculty of Pharmacy, University of Porto and Rede de Quimic e Tecnologia (REQUIMTE), Porto,Portugal (M.M.); and Burnett School of Biomedical Sciences, College of Medicine, University of Central Florida, Orlando, Florida (S.N.E.).Received June 4, 2014; accepted August 25, 2014 ABSTRACTIt has been suggested that there is a link between epinephrine adrenoceptor localization by immunohistochemistry. Epi- synthesis and the development of b2-adrenoceptor–mediated nephrine is absent in Pnmt-KO mice. The potency and the effects, but it remains to be determined whether this de- maximal effect of the b2-adrenoceptor agonist terbutaline velopment is triggered by epinephrine. The aim of this study were lower in Pnmt-KO than in wild-type (WT) mice. The was to characterize b-adrenoceptor–mediated relaxation selective b2-adrenoceptor antagonist ICI 118,551 [(6 and facilitation of norepinephrine release in the aorta of KO) mice. Catecholamines were quantified by reverse-phase tagonized the relaxation caused by terbutaline in WT but not in Pnmt-KO mice. Isoproterenol and terbutaline induced detection. Aortic rings were mounted in a myograph to concentration-dependent increases in tritium overflow in WT determine concentration-response curves to selective b1- or mice only. b2-Adrenoceptor protein density was decreased in b2-adrenoceptor agonists in the absence or presence of membrane aorta homogenates of Pnmt-KO mice, and this selective b1- or b2-adrenoceptor antagonists. Aortic rings finding was supported by immunofluorescence confocal were also preincubated with [3H]norepinephrine to measure microscopy. In conclusion, epinephrine is crucial for b2- tritium overflow elicited by electrical stimulation in the adrenoceptor–mediated vasodilation and facilitation of nor- presence of increasing concentrations of nonselective b- or epinephrine release. In the absence of epinephrine, b2- selective b2-adrenoceptor agonists. b2-Adrenoceptor protein adrenoceptor protein density was decreased in aorta cell density was evaluated by Western blotting and b2- membranes, thus potentially hindering its functional activity.
canine adrenal medulla, norepinephrine is the predominantamine, whereas in adults, epinephrine predominates (Paiva In contrast to adults, human neonates have plasma et al., 1994). On the other hand, Gootman et al. (1981) showed concentrations of norepinephrine higher than those of that b-adrenoceptor–mediated vascular relaxation is imma- epinephrine (Eliot et al., 1980). Accordingly, in the newborn ture in neonatal swine. Thies et al. (1986) also founda diminished response to the nonselective b-adrenoceptor This work was supported by grants from Fundação Professor Ernesto agonist isoproterenol in human neonatal lymphocytes com- Morais [2012, Porto, Portugal] and Porto University [Grant IJUP2011-219].
pared with adults. Paiva et al. (1994) also observed is The work performed at the Advanced Microcopy and Imaging Center of Unit for Multidisciplinary Investigation in Biomedicine was supported by Fundação a parallel time course between the postnatal increase in the para a Ciência e a Tecnologia [Grants REEQ/1264/SAU/2005, PEst-OE/SAU/ epinephrine content in the adrenal medulla and the de- UI0215/2011, and Pest-OE/SAU/UI0215/2014].
velopment of b2-adrenoceptor–mediated smooth muscle ABBREVIATIONS: CGP 20712 A, 1-[2-((3-carbamoyl-4-hydroxy)phenoxy)ethylamino]-3-[4-(1-methyl-4-trifluoromethyl-2-imidazolyl)phenoxy]-2-propanoldihydrochloride; CL 316243, 5-[(2R)-2-[[(2R)-2-(3-chlorophenyl)-2-hydroxyethyl]amino]propyl]-1,3-benzodioxole-2,2-dicarboxylic acid; Emax, maximal effect;ICI 118,551, (6)-erythro-(S*,S*)-1-[2,3-(dihydro-7-methyl-1H-inden-4-yl)oxy]-3-[(1-methylethyl)amino]-2-butanol hydrochloride; PBS, phosphate-buffered saline;pEC50, negative logarithm of the molar concentration causing 50% of Emax; Pnmt, phenylethanolamine-N-methyltransferase; Pnmt-KO, phenylethanolamine- N-methyltransferase-knockout; RIPA, radioimmunoprecipitation assay; S1, evoked tritium overflow; TBS, Tris-buffered saline; WT, wild-type.
Moreira-Rodrigues et al.
relaxation and facilitation of norepinephrine release by performance liquid chromatography column for separation of norepi- sympathetic nerve stimulation in the canine saphenous vein.
nephrine and epinephrine, which were quantified by electrochemical In addition, epinephrine is the only biogenic catecholamine detection, as previously described (Paiva et al., 1994; Moreira- that has affinity for b Rodrigues et al., 2009). The detection limit is between 350 and 1000 2-adrenoceptors at physiologically relevant concentrations, whereas both epinephrine and Postjunctional Functional Studies.
norepinephrine are potent Pnmt-KO and WT mice b1-adrenoceptors agonists (Lands aortas were placed in Krebs-Henseleit solution bubbled with 95% O et al., 1967a,b).
Thus, it was suggested that there is a link between 2, cut in rings (1–2 mm) and mounted in a myograph (DMT, Aarhus, Denmark). Each aorta ring was allowed to stabilize for 1 epinephrine and the development of b2-adrenoceptor–medi- hour. Afterward, optimal resting tension was settled using a stan- ated effects (Paiva et al., 1994; Guimaraes and Moura, 2001).
dardized normalization procedure (Mulvany and Halpern, 1977).
However, it remains to be proved whether the development of Then the arteries were precontracted with phenylephrine (a1- adrenoceptor agonist) to about 60% of the maximum contraction, 2-adrenoceptor–mediated effects is triggered by epinephrine or by other causes that might simultaneously induce a level that has been shown to be optimal to obtain b-adrenocep- epinephrine production and the functional development of tor–mediated relaxation (Guimaraes, 1975). Phenylephrine was selected for precontraction of vascular rings in our experiments It has been difficult to decipher the role of epinephrine with because relaxation responses to b-adrenoceptor agonists in vivo occurunder tonic constriction caused by a-adrenoceptor stimulation. Both the commonly used adrenal medullectomy because this in animals and in humans, changes in the balance of opposing a- and procedure can damage the adrenal cortex, altering the release b-adrenoceptor–mediated responses result in alterations of vascular of corticosteroids, and it also removes the release of other tone in vivo (Landau et al., 2002). No differences were observed adrenal amines and peptides, such as norepinephrine, between the two experimental groups concerning the degree of chromogranin A, catestatin, and neuropeptide Y (Harrison contraction induced by the concentration of phenylephrine used (0.3 and Seaton, 1966). An alternative approach is the use of mM; data not shown). Finally, concentration-response curves to dobutamine (selective b1-adrenoceptor agonist) and terbutaline to block epinephrine synthesis in vivo (Bondinell et al., 1983), (selective b2-adrenoceptor agonist) were obtained in the absence or but most of them also inhibit monoamine oxidase (Mefford presence of CGP 20712 A (1-[2-((3-carbamoyl-4-hydroxy)phenoxy) et al., 1981) and a-adrenoceptors (Feder et al., 1989). These 2-propanol dihydrochloride; selective b drawbacks for the elucidation of the specific role of epineph- 1-adrenoceptor antagonist; 40 nM) or ICI 118,551 [(6)-erythro-(S*,S*)-1-[2,3-(dihydro-7-methyl-1H- rine in the development of b2-adrenoceptor subtype are avoided by doing experiments in an epinephrine-deficient selective b2-adrenoceptor antagonist; 14 nM], respectively. The pKD animal model generated by knocking out the Pnmt gene values of terbutaline for binding to b2- and b1-adrenoceptors are (Ebert et al., 2004, 2008; Sharara-Chami et al., 2010).
around 5.62 and 3.82, respectively (Baker, 2005). Concentration- Therefore, the aim of this study was to characterize the role response curves to CL 316243 (5-[(2R)-2-[[(2R)-2-(3-chlorophenyl)-2- of epinephrine on b-adrenoceptor–mediated aorta relaxation and facilitation of norepinephrine release from sympathetic selective b3-adrenoceptor agonist) were not obtained (although nerve endings using Pnmt-knockout (Pnmt-KO) and wild-type attempt was made). The force calibration process used weight and the transducer detected 1 g [9.81millinewtons (mN) of force). Q: 2 Isometric contractions or relaxations were recorded as changes inthe millinewtons of tension.
Materials and Methods Prejunctional Functional Studies. As previously described by Trendelenburg et al. (2000), the aortas were preincubated for 30 Animals. All animal care and experimental protocols were carried minutes with [3H]norepinephrine and then placed in superfusion out in accordance with the Guide for the Care and Use of Laboratory chambers between platinum electrodes. Perifusion started at t 5 0, Animals as adopted and promulgated by the US National Institutes of and each preparation was challenged with seven periods of electrical Health and were approved by the Institute of Biomedical Sciences stimulation (1 millisecond width, 80 mA, 120 pulses at 3 Hz). The first Abel Salazar, University of Porto, Portugal, ethics committee (project stimulation period (S0) was delivered at t 5 30 minutes of perifusion no. 020/2012). The Pnmt-KO mice (Pnmt2/2) were produced by and was not used for determination of tritium overflow. The disruption of Pnmt locus by insertion of Cre-recombinase in exon 1 subsequent stimulation periods (S1–S6) were applied at t 5 58, 76, (Ebert et al., 2004). A couple of Pnmt-KO mice were kindly provided 94, 112, 130, and 148 minutes. Cocaine (26 mM; inhibitor of by S.N.E., and animals were bred in our conventional vivarium. The norepinephrine reuptake) and phentolamine (1 mM; nonselective presence of the Pnmt2/2 allele was verified by polymerase chain a-antagonist) were present in the perifusion fluid throughout the reaction of ear DNA. The animals were kept under controlled experiment. Isoproterenol and terbutaline were added to the environmental conditions (12 hour light/dark cycle, room temperature perifusion fluid at increasing concentrations (0.1 nM to 1 mM and 1 23 6 1°C, humidity 50%, autoclaved drinking water, and mice nM to 10 mM, respectively), 12 minutes before S2–S6. At the end of the breeding diet 4RF25/I; Ultragene, Porto, Portugal) and housed with perifusion period, tissues were placed in perchloric acid (0.2 M).
the respective litter. Pnmt-KO (129x1/SvJ, n 5 40) and WT (129x1/ Radioactivity was measured by liquid scintillation counting (liquid SvJ, n 5 36) male mice (8–12 weeks old) were killed by cervical scintillation counter 1209 Rackbeta; LKB Wallac, Turku, Finland) in dislocation under anesthesia (isoflurane 100%, 200 ml by inhalation).
the perifusate or tissue extract after the addition of scintillation Blood was collected and centrifuged (4°C, 3000g, 15 minutes) for mixture (Optiphase HiSafe 3; LKB, Loughborough, Leics, UK). The plasma separation. The aorta and the left adrenal gland were rapidly spontaneous outflow of tritium was calculated as a fraction of the removed and weighed and then placed in vials containing 0.3 ml of tritium content of the tissue at the onset of the respective collection perchloric acid (0.2 M) and stored at –80°C until used for period (fractional rate; min21). The overflow elicited by electrical quantification of catecholamines. Alternatively, the aortas were stimulation was expressed as a percentage of tritium content of the dissected, removed, and used for functional or molecular studies.
tissue at the time of stimulation. Percentage changes of Sn S121 ratios Quantification of Catecholamines. Alumina extracts from induced by drugs added after S1 were calculated. Finally, plasma and tissue samples were injected in reverse-phase high- b2-Adrenoceptors in Pnmt-KO Mice concentration-response curves to isoproterenol and terbutaline were hydrochloride, terbutaline hemisulfate salt, CGP 20712 A methane- sulfonate salt, ICI 118,551 hydrochloride, R-(2)-phenylephrine Protein Quantification. Three mice aortas in each sample were hydrochloride, and CL 316243 were purchased from Sigma-Aldrich Q:3 homogenized in radioimmunoprecipitation assay (RIPA) buffer (65 (St Louis, MO). Cocaine hydrochloride was obtained from Uquipa mM Tris-HCl, pH 7.4; 154 mM NaCl; 10 M Na2EDTA; 1% IGEPAL; 6 (Lisbon, Portugal), and (2)-[7-3H]norepinephrine (specific activity mM sodium deoxycholate; 1 mM phenylmethyl sulfonyl fluoride; 1 mM 14.9 Ci mmol21, 1 mCi ml21) was purchased from PerkinElmer NaF; 1 mM Na3VO4; 5 mg/ml leupeptin; 5 mg/ml aprotinin; 5 mg/ml (Waltham, MA).
pepstatin) and ultracentrifuged (4°C, 100,000g, 65 minutes). The Statistical Analysis. Concentration-response curves were ad- pellets were suspended in RIPA buffer, sonicated, and then collected justed to data by nonlinear regression analysis using GraphPad for protein quantification (membrane fraction of aorta). Protein Prism statistics software package (GraphPad Software Inc., La Jolla, concentration was determined using a protein assay kit (Bio-Rad CA). The Emax (maximal effect), EC50 , and pEC50 (negative logarithm Laboratories, Hercules, CA) using bovine serum albumin as standard.
of the molar concentration causing 50% of Emax) were estimated for Western Blot. Membrane fractions of aorta homogenates were each curve. Results are arithmetic means 6 S.E.M. of values for the diluted with RIPA buffer and then with 6:1 sample buffer (0.35 M indicated number of determinations. Unless stated otherwise, Q:4 Tris-HCl, pH 6.8; 4% SDS; 30% glycerol; 9.3% dithiothreitol; 0.01% statistical analysis was done by the two-tailed t test. Statistical bromophenol blue) and boiled at 95°C for 5 minutes. Samples (40 mg) analysis of the Sn S121 ratios was done by the Mann-Whitney test. P were separated by SDS-PAGE with 10% polyacrylamide gel and then , 0.05 was assumed to denote a significant difference.
transferred onto nitrocellulose membranes (Bio-Rad Laboratories).
Blots were blocked for 1 hour with 5% nonfat dry milk in Tris-buffered Q:5 saline (TBS), incubated with a rabbit polyclonal anti–b2-adrenoceptor antibody (1:125; Santa Cruz Biotechnology, Dallas, TX) in 2.5% nonfatdry milk in TBS/Tween 20, overnight, at 4°C, then washed and Epinephrine and Norepinephrine Content in Adre- incubated with an IRDye 800 goat anti-rabbit secondary antibody (1: nal Glands. Epinephrine content was baseline levels in the 10,000; Rockland, Gilbertsville, PA) for 1 hour at room temperature.
adrenal glands and plasma of Pnmt-KO mice when compared The membranes were then washed and imaged by scanning at both with WT mice, and it was below the detection level in the 700 nm (for detection of western blot protein standard molecular aorta samples of both groups (Table 1). In contrast, the weight; Precision Plus Protein Standard, Bio-Rad or NZY Color norepinephrine content of the adrenal gland was higher in Protein Marker II; NZYtech, Lisbon, Portugal) and 800 nm (for IRDye800 goat anti-rabbit secondary antibody detection) by fluorescence Pnmt-KO than in WT mice; however, norepinephrine levels in detection method, with an Odyssey Infrared Imaging System (LI-COR plasma and aorta were similar between the groups (Table 1).
Biosciences, Lincoln, NE). Following this, the immunoblots were washed with mild stripping solution (to remove previous primary and in Aorta Rings. In aortas previously contracted with secondary antibodies; 1% tween 20, 0.1% SDS, 200 mM glycine, pH phenylephrine, terbutaline (Fig. 1A) and dobutamine (Fig.
2.2), blocked overnight with 5% non-fat dry milk in TBS, and 1B) caused concentration-dependent relaxations in both incubated with a mouse anti–b-actin antibody (1:10,000; Santa Cruz Pnmt-KO and WT mice. The potency (EC50, 29.00 6 6.10 Biotechnology) in 2.5% nonfat dry milk in TBS/Tween 20 for 1 hour at versus 5.20 6 2.80, mM; n 5 5–6) and maximal response (7.0 6 room temperature. Finally, they were washed and incubated with an 2.4 versus 17.7 6 3.3, mN mg21; n 5 5–6) to terbutaline were Alexa Fluor 680 goat anti-mouse secondary antibody (1:10,000; lower in Pnmt-KO than in WT mice (Fig. 1A). To further Invitrogen, Eugene, OR) for 1 hour, washed again, and imaged byscanning at 700 nm (for Western blot protein standard and Alexa confirm the results obtained, we performed the concentration- Fluor 680 goat anti-mouse secondary antibody detection) (Moreira- response curve of terbutaline in the presence of a b2- Rodrigues et al., 2010).
adrenoceptor antagonist. ICI 118,551 antagonized the effect Immunohistochemistry. Aortas were fixed in 4% paraformalde- of terbutaline in WT (Fig. 2A) but not in Pnmt-KO mice (Fig.
hyde in phosphate-buffered saline (PBS) for 6 hours. Fixed tissue was 2B). The potency (EC50, 0.18 6 0.05 versus 0.22 6 0.07, mM; n washed and cryoprotected overnight with a solution containing 20% 5 6–8) and maximum response (13.8 6 3.0 versus 20.0 6 4.2, anhydrous glycerol (dissolved in 0.1 M phosphate buffer) and frozen.
mN mg21; n 5 6–8) to dobutamine were not significantly Then the tissue was sectioned (16 mm), and the tissue sections were different (P . 0.05) between experimental groups (Fig. 1B).
incubated with a blocking buffer solution (10% fetal bovine serum, 1% CGP 20712 A antagonized the effect of dobutamine in both bovine serum albumin, 0.3% Triton X-100, 0.025% NaN3 in PBS) for WT (Fig. 2C) and Pnmt-KO (Fig. 2D) mice; no differences were 60 minutes with constant stirring. Subsequently, samples wereincubated overnight (4°C) with a rabbit polyclonal anti–b2-adreno- ceptor antibody (1:50; Santa Cruz Biotechnology) diluted in dilutionbuffer solution (5% fetal bovine serum, 0.5% bovine serum albumin, 0.3% Triton X-100, 0.025% NaN Epinephrine and norepinephrine content in the adrenal gland, aorta, and 3 in PBS). Tissue sections were then washed and incubated for 90 minutes (at room temperature in the plasma in Pnmt-KO and WT mice dark) with an anti-rabbit secondary antibody Alexa Fluor 488 (1:1500; Values are means 6 S.E.M. of 5–12 experiments per group.
Molecular Probes, Eugene, OR) diluted in dilution buffer solution.
Negative controls were performed using the secondary antibody alone. Finally, samples were washed and mounted on optical-quality Epinephrine (nmol mg21) glass slides. Vectashield with DAPI (49,6-diamidino-2-phenylindole) Norepinephrine (nmol mg21) was used as mounting media (Vector Laboratories, Burlingame, CA).
Observations and analyses were performed with a laser-scanning Epinephrine (pmol mg21) confocal microscope (FV1000; Olympus Fluoview, Tokyo, Japan) Norepinephrine (pmol mg21) (Carneiro et al., 2014).
Epinephrine (pmol ml21) Drugs. Isoflorane 100%, Isoflo, was obtained from Abbott labora- Norepinephrine (pmol ml21) tories (Queenborough, UK). (2)-Epinephrine (1)-bitartrate salt, L-(2)-norepinephrine (1)-bitartrate salt monohydrate, isoproterenol ND, not detectable, below detectable limit; Pnmt-KO, phenylethanolamine-N- methyltransferase-knockout mice; WT, wild-type mice.
*Significantly different from correspondent values in WT mice (P , 0.05).
Moreira-Rodrigues et al.
Fig. 1. (A) Terbutaline (b2-adrenoceptor agonist) and (B) dobutamine (b1-adrenoceptor agonist) concentration- response curves (relaxation in percentage of maximal relaxation) of phenylephrine (a1-adrenoceptor agonist) precontracted aortas in Pnmt-KO and WT mice. maxR, maximum relaxation. Each curve point represents the mean of 5–8 experiments per group and error bars represent S.E.M. *Significantly different from corre- spondent values in WT mice (P , 0.05).
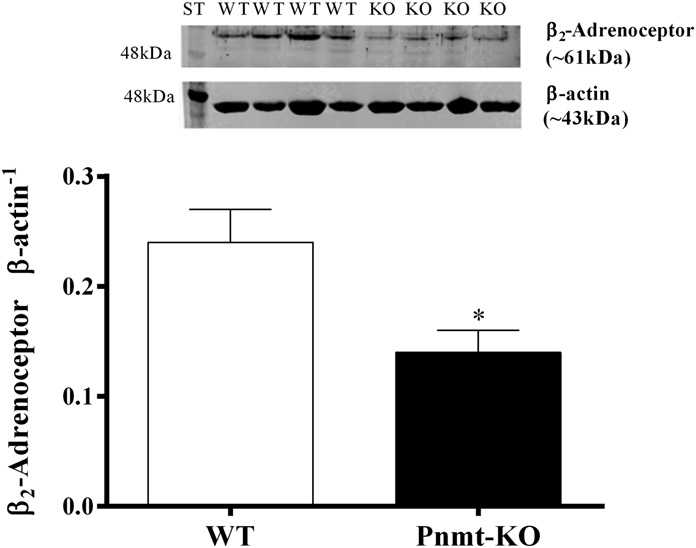
observed in pA2 values between the two groups (8.1 6 0.2 Quantification and Visualization of b2-Adrenoceptors versus 8.0 6 0.5, respectively). The b3-adrenoceptor agonist in Aorta. The protein density of b2-adrenoceptors (∼61 kDa) CL 316243 (10–10,000 nM) failed to cause vasorelaxation of in membrane aorta homogenates was significantly (P , 0.05) mice aortas (data not shown).
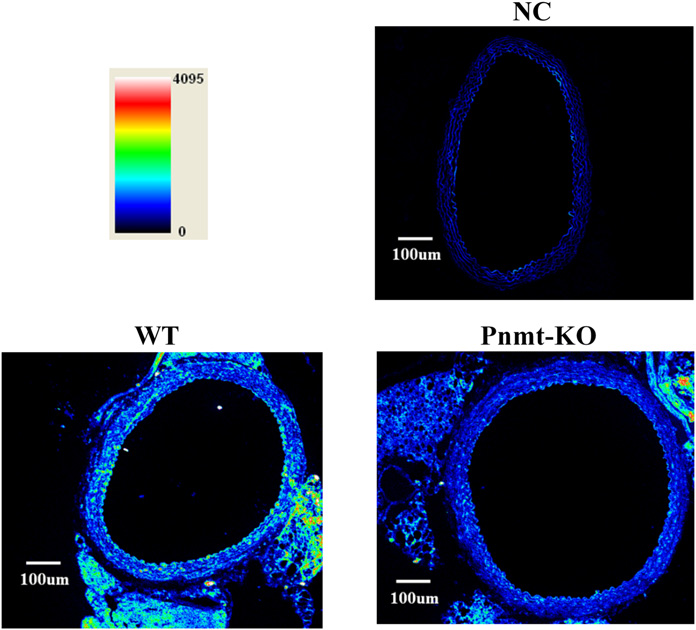
lower in Pnmt-KO compared with WT mice (Fig. 4). This Prejunctional b-Adrenoceptor–Mediated Responses finding was also evidenced by immunofluorescence confocal in Aorta Rings. No differences were found between Pnmt- microscopy (Fig. 5). In WT mice aorta rings, the b2-adreno- KO and WT mice in basal conditions: tissue accumulation of ceptor immunofluorescent labeling is concentrated mainly in tritium (430.50 6 44.95 versus 404.19 6 26.22, mmol g21; n the media layer (where smooth muscle cells are the most 5 7 to 8), fractional rate of loss (4.26 ! 1023 6 0.50 ! 1023 abundant cell type) but also in the endothelium. b2-Adreno- versus 3.89 ! 1023 6 0.13 ! 1023, min21; n 5 7–8) or ceptor immunoreactivity was decreased in aorta rings from evoked overflow of tritium induced by the control stimula- Pnmt-KO mice (341 6 37 versus 190 6 10, arbitrary tion (S1, 0.43 6 0.04 versus 0.32 6 0.04, % of tritium fluorescence intensity units; n 5 3; Fig. 5). The residual content; n 5 7–8). In the aorta of WT mice, both fluorescence labeling observed in the absence of the primary isoproterenol (Fig. 3A) and terbutaline (Fig. 3B) increased antibody (negative control) is due to autofluorescent elastic the overflow of tritium elicited by electrical stimulation in fibers (Fig. 5).
a concentration-dependent manner, with a pEC50 of 9.36 6 0.55 (n 5 7–8) and a maximal effect of 167.3 6 37.5% (n 57–8) for isoproterenol and a pEC50 of 7.65 6 0.94 (n 5 6) and a maximal effect of 173.3 6 31.3% (n 5 6) for terbutaline.
This facilitatory effect of isoproterenol and terbutaline on Our results show that epinephrine-deficient mice do not norepinephrine release was absent in Pnmt-KO mice (Fig.
develop aorta b2-adrenoceptor–mediated responses both at 3). In Pnmt-KO mice, curves failed to converge to a sigmoi- a postjunctional level (b2-adrenoceptor–dependent relaxa- dal equation (Fig. 3).
tion) and at a prejunctional level (facilitation of norepineph-rine release from sympathetic nerve endings), strengthening Fig. 2. (A and B) Terbutaline (b2-adrenoceptor agonist) concentration-response curves in the absence or pres- ence of ICI 118,551 (b2-adrenoceptor antagonist) and (C and D) dobutamine (b1-adrenoceptor agonist) concen- tration-response curves in the absence or presence of CGP 20712 A (b1-adrenoceptor antagonist), in (A and C) WT and (B and D) Pnmt-KO mice, of phenylephrine (a1- adrenoceptor agonist) precontracted aortas. maxR, maximum relaxation. Each curve point represents the mean of 5 experiments per group and error bars represent S.E.M.*Significantly different from correspon- dent values in respective mice group (P , 0.05).
b2-Adrenoceptors in Pnmt-KO Mice
Fig. 3. (A) Isoproterenol (b-adrenoceptor agonist) and (B) terbutaline (b2-adrenoceptor agonist) evoked tritium overflow of aortas in Pnmt-KO and WT
mice. In Pnmt-KO mice, the curve failed to converge to sigmoidal equation. Each curve point represents the mean of 7 to 8 experiments per group and
error bars represent S.E.M. *Significantly different from correspondent values in WT mice (P , 0.05).
the hypothesis that epinephrine is critical for the functional
20712 A (a b1-adrenoceptor antagonist) antagonized the
development of b2-adrenoceptor–mediated responses.
effect of dobutamine with similar potency in both groups.
The Pnmt-KO mouse is an epinephrine-deficient mouse
Thus, our results suggest that both b1- and b2-adrenoceptors
model generated by knocking out the Pnmt gene (Ebert et al.,
mediate aortic relaxation in WT mice but that only b1-
2004, 2008; Sharara-Chami et al., 2010). The absence of Pnmt
adrenoceptors are operative in the absence of epinephrine,
mRNA expression alters epinephrine biosynthesis. Accord-
as happens in Pnmt-KO mice. This might explain why Pnmt-
ingly, we found only baseline levels of epinephrine in the
KO mice show normal basal blood pressure values, but blood
adrenal medulla and plasma of Pnmt-KO mice. Our results
pressure dramatically increases during treadmill exercise
agree with those of Sun et al. (2008) showing that epinephrine
compared with WT mice (Bao et al., 2007). In Pnmt-KO mice
is absent from the adrenal gland and plasma of Pnmt-KO
at rest, b1-adrenoceptors may be sufficient to produce
mice, whereas the norepinephrine content of the adrenal
vasodilation and control blood pressure. One can speculate
glands is significantly increased. This result might be due to
that under stressful conditions, this mechanism might not be
an upstream accumulation of norepinephrine that would
enough because b2-adrenoceptor–mediated vasodilator re-
normally be methylated to epinephrine.
sponse is blunted, and then blood pressure rises. In
In conduit arteries (thoracic aorta and carotid artery) of
agreement with this hypothesis is the fact that b2-adreno-
mice, both b1- and b2-adrenoceptors mediate smooth muscle
ceptor KO mice also have a normal basal resting blood
relaxation (Chruscinski et al., 1999, 2001; Rohrer et al., 1999).
pressure and become hypertensive during exercise (Chrus-
We chose the mouse aorta because 1) it contains postjunc-
cinski et al., 1999).
tional relaxing b2-adrenoceptors, 2) it is easy to mount in the
Conversely, we did not observe b3-adrenoceptor–mediated
myograph to evaluate b2-adrenoceptor–mediated relaxation,
vasorelaxation induced by CL 316243 in WT mice, which is in
and 3) it has enough nerve terminals to evaluate prejunc-tional b-adrenoceptor–mediated responses.
Our results showed that dobutamine (b1-adrenoceptor
agonist) and terbutaline (b2-adrenoceptor agonist) caused
concentration-dependent relaxation of aorta rings precon-tracted with phenylephrine in both WT and Pnmt-KO mice. InWT mice, terbutaline caused aorta relaxation at b2-selective
concentrations, whereas in Pnmt-KO mice, very high (non-selective) concentrations of terbutaline were required toproduce relaxation. Actually, the potency and the maximalresponse to b2-adrenoceptor stimulation by terbutaline were
lower in Pnmt-KO mice than in WT mice. In addition, the b2-
adrenoceptor antagonist ICI 118,551 failed to modify therelaxing effect of terbutaline in Pnmt-KO mice, contrary tothat observed in WT mice. Overall, these results suggest a lossof b2-adrenoceptor–mediated relaxation in the aorta of Pnmt-
Q:6 KO compared with WT mice. In agreement with our results is
the fact that after adrenalectomy impaired formation of high-affinity myocardial b-adrenoceptor complexes was observed(Davies et al., 1981), and after adrenal demedullationa decrease in b-adrenoceptor cardiac density was observedin rats (Tumer et al., 1990).
Fig. 4. Semiquantification of b2-adrenoceptor protein (∼61 kDa) in
On the other hand, no differences were observed in b
membrane aorta homogenates, in Pnmt-KO and WT mice. Immunoblot
quantification is normalized with b-actin protein. Data shown are
–mediated aorta relaxation to dobutamine
representative immunoblots; bars represent means of 7–10 experiments
between the two groups since the potency and the maximal
per group, and error bars represent S.E.M. ST, Western blot protein
response to dobutamine were similar. In addition, CGP
standard. *Significantly different from correspondent values in WT mice
Moreira-Rodrigues et al.
norepinephrine release facilitation on b2-adrenoceptor acti-
vation (Guimaraes and Moura, 2001). Signaling complexesare located in specialized membrane compartments and areenriched in components of the signal transduction cascade,including G proteins and effector molecules. Thus, thesecomplexes may act as a scaffold promoting the interaction ofspecific signaling proteins (Galbiati et al., 2001). In Pnmt-KOmice, one possible explanation for the presence of reduced b2-
adrenoceptor protein density (41.7 6 8.3%) and the absence ofb2-adrenoceptor function could be a differential association ofb2-adrenoceptors to the signaling complexes in the mem-
brane, which could alter the spatial relationship of b2-
adrenoceptors with the associated signaling proteins, therebyleading to an abrupt decrease in activation of the proteineffectors.
The highest b2-adrenoceptor immunofluorescent labeling
found in aorta rings was in the media layer, where smoothmuscle cells are most abundant. Immunolocalization studiesalso revealed the presence of b2-adrenoceptors in the
endothelial lining of mice aortic rings. Given that b2-
adrenoceptors located in vascular endothelial cells may
Fig. 5. Representative confocal micrographs of b
regulate NO release (Conti et al., 2013), one cannot exclude
2-adrenoceptor immuno-
reactivity of aortas in Pnmt-KO and WT mice. The images are shown as
that relaxation of mouse aortic rings produced by b2-
a pseudocolor spectral display. b2-Adrenoceptor protein immunoreactivity
adrenoceptor agonists involves this mechanism.
is represented by the signal intensity of a standardized color pallet, dark
The results from this study agree with the fact that the time
blue is low intensity, and red is high intensity. Similar results were
obtained in two individual experiments. Image scale bar is 100 mm. The
course of postnatal increase in the epinephrine content of the
negative control (NC) is secondary antibody alone.
adrenal medulla positively correlates with the development ofb2-adrenoceptor–mediated effects, as previously described in
agreement with the fact that in aortas from
the canine saphenous vein (Paiva et al., 1994). This view is
tor double-knockout mice, the
supported by the diminished response of b-adrenoceptors
b-agonist isoproterenol does not
cause any relaxation (Chruscinski et al., 2001). These findings
in lymphocytes from human newborns compared with adults
(Thies et al., 1986), and lymphocytes almost exclusively
b3-adrenoceptors do not contribute to the
adrenoceptor-mediated vasodilation in mice aorta.
express adrenoceptors of the b2-subtype (Sanders, 2012).
In the aorta of WT mice, the facilitatory effect of iso-
Interestingly, it was found that treatment of newborn rats
proterenol on norepinephrine release was similar to that
with b2-adrenoceptor agonists caused a surprising sensitiza-
found by other authors in mice spleen and atria (Trendelen-
tion of b-adrenoceptors instead of evoking desensitization
burg et al., 2000). To our knowledge, data from this study
(Kudlacz and Slotkin, 1990). Also, a single exposure of
show for the first time that prejunctional
newborn rats to terbutaline induces a strong sensitization of
b-adrenoceptors, in
particular of the
2-adrenoceptor–mediated stimulation of adenylyl cyclase
2 subtype, are present in the mouse aorta.
In addition, we report here the absence of
activity in the brainstem and cerebellum (Slotkin and Seidler,
2006). Epinephrine is the only biogenic catecholamine that
–mediated effect on norepinephrine release in Pnmt-KO
mice. In agreement with our results, it had been previously
has a good affinity for b2-adrenoceptors (Lands et al., 1967a,
shown that carteolol (nonselective
b), and it is conceivable that it can act similarly, causing
does not inhibit [3H]norepinephrine release in a concentra-
sensitization of b2-adrenoceptors. Since Pnmt-KO mice were
tion-dependent manner induced by nerve stimulation from
never exposed to epinephrine, b2-adrenoceptor function does
pulmonary arteries in guinea pigs subjected to adrenalectomy
not appear to develop efficiently.
or adrenodemedullation, contrary to sham-operated animals
One limitation of our experimental design is the limited
(Misu et al., 1989). On the other hand, putative prejunctional
selectivity of terbutaline and dobutamine at b2- and b1-
adrenoceptors, respectively (Ruffolo et al., 1984; Young et al.,
b1-adrenoceptor–mediated effects were not evaluated because
in all tissues tested so far, presynaptic
2002; Baker, 2005). This prevents us from using higher
b-adrenoceptors are of
nonselective concentrations of those agonists because the
b2 subtype (Kahan and Hjemdahl, 1987; Molderings et al.,
1988; Trendelenburg et al., 2000; Todorov et al., 2001).
mouse aorta relaxes to activation of both subtypes of
Although compensatory changes may occur, the presence of
In conclusion, epinephrine is crucial for the development
b1- or b3-adrenoceptors seems rather unlikely
since we did not observe any
b-adrenoceptor induced effect in
2-adrenoceptors and associated b2-adrenoceptor–medi-
the release of norepinephrine using isoproterenol (a non-
ated aorta vasodilation and facilitation of norepinephrine
release by sympathetic nerves. In the absence of epineph-
2-adrenoceptor protein density was decreased in aorta
2-adrenoceptor protein density observed in
aorta of Pnmt-KO mice may be correlated with decreased
cell membranes, thus potentially hindering its functional
coupling to stimulatory G protein and lower cAMP levels,
which may justify impairments of vasorelaxation and of
b2-Adrenoceptors in Pnmt-KO Mice
Landau R, Dishy V, Wood AJ, Stein CM, and Smiley RM (2002) Disproportionate
The authors thank Vânia Monteiro for technical support.
decrease in alpha- compared with beta-adrenergic sensitivity in the dorsal hand
vein in pregnancy favors vasodilation. Circulation 106:1116–1120.
Lands AM, Arnold A, McAuliff JP, Luduena FP, and Brown TG, Jr (1967a) Differ-
Authorship Contributions
entiation of receptor systems activated by sympathomimetic amines. Nature 214:
Participated in research design: Moreira-Rodrigues, Moura.
Lands AM, Luduena FP, and Buzzo HJ (1967b) Differentiation of receptors re-
Conducted experiments: Moreira-Rodrigues, Graça, Ferreira,
sponsive to isoproterenol. Life Sci 6:2241–2249.
Afonso, Serrão, Manuela Morato, Ferreirinha, Moura.
Mefford IN, Roth KA, Gilberg M, and Barchas JD (1981) In vivo intraneuronal MAO
Performed data analysis: Moreira-Rodrigues, Graça, Ferreira,
inhibition in rat brain SKF 64139, comparison to other potent PNMT inhibitors.
Eur J Pharmacol 70:345–353.
Ferreirinha, Correia-de-Sá, Moura.
Misu Y, Kuwahara M, Amano H, and Kubo T (1989) Evidence for tonic activation of
Wrote or contributed to the writing of the manuscript: Moreira-
prejunctional beta-adrenoceptors in guinea-pig pulmonary arteries by adrenaline
Rodrigues, Graça, Morato, Correia-de-Sá, Ebert, Moura.
derived from the adrenal medulla. Br J Pharmacol 98:45–50.
Molderings G, Likungu J, Zerkowski HR, and Göthert M (1988) Presynaptic beta 2-
adrenoceptors on the sympathetic nerve fibres of the human saphenous vein: no
evidence for involvement in adrenaline-mediated positive feedback loop regulating
Baker JG (2005) The selectivity of beta-adrenoceptor antagonists at the human
noradrenergic transmission. Naunyn Schmiedebergs Arch Pharmacol 337:
beta1, beta2 and beta3 adrenoceptors. Br J Pharmacol 144:317–322.
Bao X, Lu CM, Liu F, Gu Y, Dalton ND, Zhu BQ, Foster E, Chen J, Karliner JS,
Moreira-Rodrigues M, Quelhas-Santos J, Serrão P, Fernandes-Cerqueira C, Sam-
and Ross J, Jr, et al. (2007) Epinephrine is required for normal cardiovascular
paio-Maia B, and Pestana M (2010) Glycaemic control with insulin prevents the
responses to stress in the phenylethanolamine N-methyltransferase knockout
reduced renal dopamine D1 receptor expression and function in streptozotocin-
mouse. Circulation 116:1024–1031.
induced diabetes. Nephrol Dial Transplant 25:2945–2953.
Bondinell WE, Chapin FW, Frazee JS, Girard GR, Holden KG, Kaiser C, Maryanoff
Moreira-Rodrigues M, Sampaio-Maia B, and Pestana M (2009) Renal dopaminergic
C, Perchonock CD, Gessner GW, and Hieble JP, et al. (1983) Inhibitors of phe-
system activity in rat remnant kidney up to twenty-six weeks after surgery. Life
nylethanolamine N-methyltransferase and epinephrine biosynthesis: a potential
Sci 84:409–414.
source of new drugs. Drug Metab Rev 14:709–721.
Mulvany MJ and Halpern W (1977) Contractile properties of small arterial resistance
Carneiro I, Timóteo MA, Silva I, Vieira C, Baldaia C, Ferreirinha F, Silva-Ramos M,
vessels in spontaneously hypertensive and normotensive rats. Circ Res 41:19–26.
and Correia-de-Sá P (2014) Activation of P2Y6 receptors increases the voiding
Paiva MQ, Moura D, Vaz-da-Silva MJ, and Guimarães S (1994) Postnatal de-
frequency in anaesthetized rats by releasing ATP from the bladder urothelium. Br
velopment of vascular beta-adrenoceptor-mediated responses and the increase in
J Pharmacol 171:3404–3419.
the adrenaline content of the adrenal gland have a parallel time course. Naunyn
Chruscinski A, Brede ME, Meinel L, Lohse MJ, Kobilka BK, and Hein L (2001)
Schmiedebergs Arch Pharmacol 350:28–33.
Differential distribution of beta-adrenergic receptor subtypes in blood vessels of
Rohrer DK, Chruscinski A, Schauble EH, Bernstein D, and Kobilka BK (1999) Car-
knockout mice lacking beta(1)- or beta(2)-adrenergic receptors. Mol Pharmacol 60:
diovascular and metabolic alterations in mice lacking both beta1- and beta2-
adrenergic receptors. J Biol Chem 274:16701–16708.
Chruscinski AJ, Rohrer DK, Schauble E, Desai KH, Bernstein D, and Kobilka BK
Ruffolo RR, Jr, Messick K, and Horng JS (1984) Interactions of three inotropic
(1999) Targeted disruption of the beta2 adrenergic receptor gene. J Biol Chem 274:
agents, ASL-7022, dobutamine and dopamine, with alpha- and beta-adrenoceptors
in vitro. Naunyn Schmiedebergs Arch Pharmacol 326:317–326.
Conti V, Russomanno G, Corbi G, Izzo V, Vecchione C, and Filippelli A (2013)
Sanders VM (2012) The beta2-adrenergic receptor on T and B lymphocytes: do we
Q:7 Adrenoreceptors and nitric oxide in the cardiovascular system. Front Physiol 4:321.
understand it yet? Brain Behav Immun 26:195–200.
Davies AO, De Lean A, and Lefkowitz RJ (1981) Myocardial beta-adrenergic recep-
Sharara-Chami RI, Joachim M, Mulcahey M, Ebert S, and Majzoub JA (2010) Effect
tors from adrenalectomized rats: impaired formation of high-affinity agonist-
of epinephrine deficiency on cold tolerance and on brown adipose tissue. Mol Cell
receptor complexes. Endocrinology 108:720–722.
Ebert SN, Rong Q, Boe S, and Pfeifer K (2008) Catecholamine-synthesizing cells in
Slotkin TA and Seidler FJ (2006) Anomalous regulation of beta-adrenoceptor sig-
the embryonic mouse heart. Ann N Y Acad Sci 1148:317–324.
naling in brain regions of the newborn rat. Brain Res 1077:54–58.
Ebert SN, Rong Q, Boe S, Thompson RP, Grinberg A, and Pfeifer K (2004) Targeted
Sun P, Bao X, Elayan H, Milic M, Liu F, and Ziegler MG (2008) Epinephrine regu-
insertion of the Cre-recombinase gene at the phenylethanolamine n-
lation of hemodynamics in catecholamine knockouts and the pithed mouse. Ann N
methyltransferase locus: a new model for studying the developmental distribu-
Y Acad Sci 1148:325–330.
tion of adrenergic cells. Dev Dyn 231:849–858.
Thies WR, Reinhardt D, Rutschke A, Kusenbach G, and Ludwig J (1986) [Postnatal
Eliot RJ, Lam R, Leake RD, Hobel CJ, and Fisher DA (1980) Plasma catecholamine
development of the sympathoadrenergic system in premature and newborn
concentrations in infants at birth and during the first 48 hours of life. J Pediatr 96:
infants]. Monatsschr Kinderheilkd 134:453–458.
Todorov LD, Clerkin R, Mihaylova-Todorova ST, Khoyi MA, and Westfall DP (2001)
Feder HH, Crowley WR, and Nock B (1989) Inhibition of guinea pig lordosis behavior
Beta2-adrenoceptor-mediated prejunctional facilitation and postjunctional in-
by the phenylethanolamine N-methyltransferase (PNMT) inhibitor SKF-64139:
hibition of sympathetic neuroeffector transmission in the guinea pig vas deferens.
mediation by alpha noradrenergic receptors. Horm Behav 23:106–117.
J Pharmacol Exp Ther 298:623–633.
Galbiati F, Razani B, and Lisanti MP (2001) Emerging themes in lipid rafts and
Trendelenburg AU, Cox SL, Schelb V, Klebroff W, Khairallah L, and Starke K (2000)
caveolae. Cell 106:403–411.
Modulation of (3)H-noradrenaline release by presynaptic opioid, cannabinoid and
Gootman PM, Gootman N, Turlapaty PD, Yao AC, Buckley BJ, and Altura BM (1981)
bradykinin receptors and beta-adrenoceptors in mouse tissues. Br J Pharmacol
Autonomic regulation of cardiovascular function in neonates. Ciba Found Symp 83:
Tumer N, Houck WT, Boehm C, and Roberts J (1990) Cardiac beta-adrenoceptor
Guimarães S (1975) Further study of the adrenoceptors of the saphenous vein of the
binding characteristics with age following adrenal demedullation. Br J Pharmacol
dog: influence of factors which interfere with the concentrations of agonists at the
receptor level. Eur J Pharmacol 34:9–19.
Young L, Bercute-Dammann A, and Weis MT (2002) Mg21 efflux from the isolated
Guimarães S and Moura D (2001) Vascular adrenoceptors: an update. Pharmacol Rev
perfused rabbit heart is mediated by two states of the beta1-adrenergic receptor.
Naunyn Schmiedebergs Arch Pharmacol 366:431–439.
Harrison TS and Seaton JF (1966) Tissue content of epinephrine and norepinephrine
following adrenal medullectomy. Am J Physiol 210:599–600.
Kahan T and Hjemdahl P (1987) Prejunctional beta 2-adrenoceptor-mediated en-
Address correspondence to: M. Moreira-Rodrigues, Laboratory of General
hancement of noradrenaline release in skeletal muscle vasculature in situ. J
Physiology, Building 2, Floor 4, Cabinet 22, ICBAS, University of Porto, R.
Cardiovasc Pharmacol 10:433–438.
Jorge Viterbo Ferreira, 228, 4050-313, Porto, Portugal. E-mail: mirodrigues@
Kudlacz EM and Slotkin TA (1990) Regulation of neonatal rat lung compliance by
beta-adrenergic receptor stimulation: effects of prenatal exposure to terbutaline or
dexamethasone. J Dev Physiol 14:307–310.
Source: http://intelisoftbd.net/user-post/wp-content/uploads/2014/09/Artigo.pdf
Microsoft powerpoint - metbionetporphyriachecked.ppt
Dr Mike Badminton Senior Lecturer/Honorary Consultant Cardiff SAS Porphyria Service (www.cardiff-porphyria.org) Department of Medical Biochemistry University Hospital of Wales and Cardiff University I Overview and Introduction II Acute Hepatic Porphyrias III Cutaneous Porphyria I. Overview The porphyrias comprise a group of disorders of the haem biosynthetic pathway that can present with acute neurovisceral
australia21.org.au
Editors Bob Douglas and Jo Wodak Trauma-related stress in AustraliaEssays by leading Australian thinkers and researchers Stress arising from trauma is affecting millions of Australians. A national conversation is required to consider how we can better manage this problem. Australia21 LtdABN: 25096242410 ACN: 096242410 PO Box 3244 Weston ACT 2611Phone: +61(0)2 62880823Email: [email protected]