Eretina.com
Randomized, Sham-Controlled Trial ofDexamethasone Intravitreal Implant in
Patients with Macular Edema Due to
Retinal Vein Occlusion
Julia A. Haller, MD,1 Francesco Bandello, MD,2 Rubens Belfort, Jr., MD,3 Mark S. Blumenkranz, MD,4Mark Gillies, MD,5 Jeffrey Heier, MD,6 Anat Loewenstein, MD,7 Young-Hee Yoon, MD,8Marie-Louise Jacques, MD,9 Jenny Jiao, PhD,10 Xiao-Yan Li, MD,10 Scott M. Whitcup, MD,10 for theOZURDEX GENEVA* Study Group To evaluate the safety and efficacy of dexamethasone intravitreal implant (DEX implant; OZURDEX, Allergan, Inc., Irvine, CA) compared with sham in eyes with vision loss due to macular edema (ME) associatedwith branch retinal vein occlusion (BRVO) or central retinal vein occlusion (CRVO).
Two identical, multicenter, masked, randomized, 6-month, sham-controlled clinical trials (each of which included patients with BRVO and patients with CRVO).
A total of 1267 patients with vision loss due to ME associated with BRVO or CRVO.
A single treatment with DEX implant 0.7 mg (n ⫽ 427), DEX implant 0.35 mg (n ⫽ 414), or sham (n ⫽ 426).
Main Outcome Measures:
The primary outcome measure for the pooled data from the 2 studies was time to achieve a ⱖ15-letter improvement in best-corrected visual acuity (BCVA). Secondary end points includedBCVA, central retinal thickness, and safety.
After a single administration, the time to achieve a ⱖ15-letter improvement in BCVA was signifi- cantly less in both DEX implant groups compared with sham (P⬍0.001). The percentage of eyes with a ⱖ15-letterimprovement in BCVA was significantly higher in both DEX implant groups compared with sham at days 30 to90 (P⬍0.001). The percentage of eyes with a ⱖ15-letter loss in BCVA was significantly lower in the DEX implant0.7-mg group compared with sham at all follow-up visits (Pⱕ0.036). Improvement in mean BCVA was greater inboth DEX implant groups compared with sham at all follow-up visits (Pⱕ0.006). Improvements in BCVA with DEXimplant were seen in patients with BRVO and patients with CRVO, although the patterns of response differed. Thepercentage of DEX implant-treated eyes with intraocular pressure (IOP) of ⱖ25 mmHg peaked at 16% at day 60(both doses) and was not different from sham by day 180. There was no significant between-group difference inthe occurrence of cataract or cataract surgery.
Dexamethasone intravitreal implant can both reduce the risk of vision loss and improve the speed and incidence of visual improvement in eyes with ME secondary to BRVO or CRVO and may be a usefultherapeutic option for eyes with these conditions.
Proprietary or commercial disclosure may be found after the references.
Ophthalmology 2010;117:1134 –1146 2010 by the American Academy of Ophthalmology. *Group members listed online in Appendix 1 (available at http://aaojournal.org). Retinal vein occlusion (RVO) is second only to diabetic larization and vision loss. Many eyes with nonischemic retinopathy as a cause of vision loss due to vascular diseases CRVO are also at risk for significant vision loss because of of the retina; it may involve the central retinal vein or 1 or macular edema (ME) and other less frequent complica- more hemicentral or branch retinal veins in various combi- It is estimated that 12% to 33% of cases of CRVO nations and with varying degrees of ischemia and hemor- that are nonischemic at the time of presentation may be- Branch retinal vein occlusion (BRVO) involving a come ischemic within 4 single vein is the most common type (prevalence of 0.6%– Macular edema is a common cause of vision loss in both 1.1%), whereas central retinal vein occlusion (CRVO) is BRVO and The pathogenesis of ME in RVO is not less common (prevalence of 0.1%– Ischemic completely understood but may result from a variety of CRVO (up to 25% of cases) is a particularly serious con- factors, including hydrostatic effects from increased venous dition with a substantial risk of secondary ocular neovascu- pressure, the presence of inflammatory cytokines (e.g., pros- 2010 by the American Academy of Ophthalmology ISSN 0161-6420/10/$–see front matter Published by Elsevier Inc.
Haller et al 䡠 Novel Dexamethasone Drug Delivery System in Treatment of RVO taglandins and interleukin-6), the dysregulation of endothe- lial tight junction or increased amounts of vascu-lar permeability factors, such as vascular endothelial growth Patients were recruited at 167 clinical sites in 24 countriesthroughout the world. Patients who were at least 18 years of age and had decreased visual acuity as a result of clinically detectable Several therapies are being investigated for the treatment ME associated with either CRVO or BRVO were recruited into of ME associated with RVO. These include laser photoco- both studies. Duration of ME (defined as the time since initial the anti-VEGF therapy and diagnosis of ME) was required to be between 6 weeks and 9 the corticosteroids triamcinolone and dexa- months in patients with CRVO and between 6 weeks and 12 Corticosteroids can help reduce many of the months in patients with BRVO. The investigator selected 1 eye per processes thought to play a role in the development of ME patient to be the study eye. If both eyes were eligible, then the eye in they have potent anti-inflammatory effects, with the shorter duration of ME was selected. Eligible patients hadto have best-corrected visual acuity (BCVA) of between 34 letters can reduce vascular permeability, inhibit fibrin deposition (20/200) and 68 letters (20/50) in the study eye and better than 34 and leukocyte movement, suppress homing and migration letters in the nonstudy eye. Retinal thickness in the central subfield of inflammatory cells, stabilize endothelial cell tight junc- (as measured by optical coherence tomography; OCT2 or OCT3) tions, and inhibit the synthesis of VEGF, prostaglandins, had to be ⱖ300 m in the study eye.
and other Intravitreal injections of the li- Key exclusion criteria included the presence of a clinically signif- pophilic corticosteroid triamcinolone acetonide have been icant epiretinal membrane, active retinal or optic disc neovasculariza- shown to produce benefits in eyes with RVO, but several tion, active or history of choroidal neovascularization, presence ofrubeosis iridis, any active infection, aphakia or anterior-chamber adverse events have been noted (with elevated intraocular intraocular lens, clinically significant media opacity, glaucoma or pressure [IOP] being the most Other current ocular hypertension requiring more than 1 medication to corticosteroids, however, have their own unique proper- control IOP in the study eye, or a history of steroid-induced IOP and may have different clinical profiles in intravitreal increase in either eye. Patients were also excluded if they had diabetic retinopathy in either eye, had any uncontrolled systemic Dexamethasone is a potent, water-soluble corticosteroid disease, were currently using or anticipating the use of systemic that can be delivered to the vitreous cavity by the dexa- steroids or anticoagulants during the study, or had any ocular methasone intravitreal implant (DEX implant; OZURDEX, condition in the study eye that, in the opinion of the investigator,would prevent a 15-letter improvement in visual acuity.
Allergan, Inc., Irvine, CA). A DEX implant is composed of abiodegradable copolymer of lactic acid and glycolic acid con-taining micronized dexamethasone. The drug-copolymer com- Randomization and Masking
plex gradually releases the total dose of dexamethasoneover a series of months after insertion into the eye through Patients were randomized to either a sham procedure or treatment a small pars plana puncture using a customized applicator with 0.35 or 0.7 mg DEX implant using a 1:1:1 allocation ratio.
system. In a recent study in eyes with persistent ME from Randomization was performed centrally (using an interactive several different causes (including RVO), DEX implant 0.7 voice response system) and stratified by the underlying cause of mg produced improvements in visual acuity, macular thick- RVO (BRVO or CRVO). The treatment investigator performed thestudy treatment procedure, evaluated the quality of the OCT scans, ness, and fluorescein leakage that were sustained for up to 6 and was responsible for the overall safety of study participants, but kept all study medication information confidential and did not This 6-month study evaluated the safety and efficacy of collect efficacy information. Patients were masked with regard to DEX implant 0.35 mg and 0.7 mg compared with a sham study treatment, and the key efficacy variables were collected and procedure in eyes with vision loss due to ME associated evaluated by follow-up investigators who were also masked with with BRVO or CRVO. The time course of the response to regard to study treatment. A central reading center (University treatment was also evaluated.
of Wisconsin Fundus Photograph Reading Center) was used toevaluate OCT measurements of central retinal thickness usingstandardized procedures and graders masked to study groupassignment.
Materials and Methods
Study Design
On day 0, the study eye was anesthetized with topical and sub- Two randomized, prospective, multicenter, masked, sham-controlled, conjunctival anesthetics and prepared according to standard clin- parallel-group clinical trials were conducted in compliance with ical practice for eyes undergoing intravitreal injection. The DEX regulatory obligations, the Declaration of Helsinki, and the insti- implant was inserted into the vitreous cavity through the pars plana tutional review board and informed consent regulations at each using a customized, single-use, 22-gauge applicator. The sham investigational site. These trials are registered at procedure followed the same protocol, including anesthetic and as NCT00168324 and NCT00168298. Two separate phase III surgical preparation, but used a needleless applicator placed clinical trials (each of which included patients with BRVO and against the conjunctiva to simulate the placement of study medi- patients with CRVO) were conducted for regulatory purposes; cation. Patients were treated with a topical ophthalmic antibiotic 4 because the study designs for the 2 trials were identical, the results times daily starting 3 days before the day of their study procedure were pooled for analysis.
(day 0) and continuing for 3 days after the procedure.
Volume 117, Number 6, June 2010 lens opacities was measured during the slit-lamp examinationusing standardized photographs.
Nonstudy treatments considered necessary for the best care of the Patients were evaluated at baseline and days 1, 7, 30, 60, 90, patient could be given at the discretion of the investigator. Doses and 180 posttreatment. Best-corrected visual acuity, IOP, biomi- of any medication that could have an effect on study outcomes croscopy, ophthalmoscopy, and adverse events were evaluated at were to remain constant throughout the study. Unless required for each study visit. Fluorescein angiography and vital signs were patient care, the use of laser/surgical treatment, topical nonsteroi- assessed at baseline and day 180, and central retinal thickness was dal anti-inflammatory agents, or intravitreal, periocular, or topical assessed at baseline, day 90, and day 180.
steroids in the study eye was prohibited during the study. The useof systemic steroids, immunosuppressants, immunomodulators, Data Analysis and Statistical Methods
anticoagulants, antimetabolites, and alkylating agents was alsoprohibited. The use of prohibited therapies was recorded as an The patient population used for the analyses of primary and escape treatment. Patients who received prohibited treatments secondary efficacy variables was the intent-to-treat population, were not required to discontinue from the study, and their efficacy which included all randomized patients. The analyses of safety and safety outcomes were included in the intent-to-treat analyses.
variables included patients who received study treatment after The data from patients who received prohibited medications that randomization and were based on the actual treatment that each were considered major protocol violations were excluded from the patient received. To confirm the robustness of the findings from the per-protocol efficacy analyses.
intent-to-treat analyses, a per-protocol analysis that excluded pa-tients with major protocol violations was also performed on key efficacy variables.
The time to reach a 15-letter improvement from baseline was The prospectively defined primary efficacy analysis for the pooled evaluated using the Kaplan–Meier method, and differences be- data from the 2 phase III studies was the time to reach a 15-letter tween the treatment groups were assessed using the log-rank test improvement from baseline BCVA. Best-corrected visual acuity comparing the cumulative response rate curves across time during was measured using a standardized Early Treatment Diabetic Ret- the 6-month study period. Categoric variables were analyzed using inopathy Study Testing was done at a standardized the Pearson chi-square test or Fisher exact test. Continuous vari- distance (4 m) under standardized lighting conditions.
ables were analyzed using analysis of variance. Pairwise between- These studies were the first designed to achieve Food and Drug group comparisons for categoric change from baseline BCVA (an Administration regulatory approval for a therapy indicated for the ordinal response) were performed using the Wilcoxon rank-sum treatment of ME due to RVO, and 2 separate phase III trials were test. The comparisons for the secondary outcome measures were required. The Food and Drug Agency requested that the primary performed at the ␣ ⫽ 0.05 significance level. All tests were outcome for the first study be the proportion of eyes achieving at least a 15-letter improvement from baseline BCVA at day 180. The Missing data were replaced by last observation carried forward agency later requested that the prospective primary outcome mea- for all BCVA analyses, except Kaplan–Meier analysis, using the sure for the second study be the time to reach a 15-letter improve- intent-to-treat population. A gatekeeping procedure was used to ment from baseline BCVA.
control the overall type I error rate at 5% for multiple comparisons Secondary efficacy analyses for the pooled data included the between groups in various efficacy variables.
assessment of the proportion of eyes achieving at least a 10-, 11-, For each of the 2 phase III studies, a sample size of 495 eyes 12-, 13-, 14-, or 15-letter improvement from baseline BCVA; the (165 per group) was estimated to provide an 81% power for proportion of eyes exhibiting ⱖ15 letters of worsening from base- detecting an 11% difference between a DEX implant treatment line BCVA; and the mean change from baseline BCVA. Subgroup group and the sham group in the proportion of eyes that achieved analyses of changes in BCVA included a prospectively planned at least a 15-letter improvement in BCVA at day 180. This calcu- analysis based on RVO diagnosis (BRVO vs. CRVO) and a post lation was based on a 2-sided chi-square test at ␣ level 0.05, hoc subgroup analysis based on duration of ME at baseline.
assuming a 9% improvement rate for sham. Accounting for an Secondary outcome measures also included central subfield estimated dropout rate of 10%, a total of 550 eyes was planned for retinal thickness measured using OCT. For the OCT analysis, images were obtained from each study eye after pupil dilation bya certified operator using an OCT2 (Carl Zeiss Meditec Inc.,Dublin, CA; 1 site only) or OCT3 (Stratus OCT, Carl Zeiss Meditec Inc.; all other sites) system. Two images were obtained atthe screening visit, and 6 images were obtained at the OCT Patients were recruited into this study between November 2004 follow-up visits (days 90 and 180); the central subfield retinal and March 2008. A total of 1267 patients (DEX implant 0.7 mg: thickness used for statistical analyses was determined by taking the n ⫽ 427; DEX implant 0.35 mg: n ⫽ 414; sham: n ⫽ 426) were average of the 6 images obtained at each follow-up visit. Material enrolled, and the majority of patients (1196/1267; 94%) completed obtained via OCT included retinal maps (especially the 6-mm day 180 There was no statistically significant between- variant and the 6 maps composing the radial pattern) and the 2 group difference with regard to the number of patients who com- align (or align/normalize) prints from the 6 to 12 o'clock and 3 to pleted or withdrew from the study before day 180. Fifteen of 1267 9 o'clock scans ("crosshairs") in the radial pattern. These materials patients (1.2%) withdrew from the study because of ocular adverse were sent to the University of Wisconsin Fundus Photograph events; 2 cases were considered to be treatment related.
Reading Center for grading. Central retinal thickness was deter- Demographic and baseline characteristics of the study popula- mined from the central 1-mm macular subfield (correlation be- tion are listed in Approximately twice as many patients tween center point thickness and central subfield thickness was had BRVO (830/1267, 66%) as CRVO (437/1267, 34%), and a minority of patients (211/1267; 17%) had duration of ME ⬍90 Safety parameters, including IOP assessments, slit-lamp biomi- days. At baseline, mean visual acuity was approximately 54 letters croscopy, ophthalmoscopy, and adverse events, were also as- (20/80) in all groups, and mean central retinal thickness was sessed. The presence of nuclear, cortical, and posterior subcapsular approximately 550 m. Ten percent of patients (131/1267) had a Haller et al 䡠 Novel Dexamethasone Drug Delivery System in Treatment of RVO Figure 1. Patient flow through the study. AE ⫽ adverse event; DEX implant ⫽ dexamethasone intravitreal implant (OZURDEX, Allergan, Inc., Irvine, CA).
history of photocoagulation, and 801 of 1267 patients (63%) had at and in an analysis of the per-protocol hypertension. There was no statistically significant difference among the 3 treatment groups with regard to any demographic or The proportion of eyes achieving at least a 15-letter improve- ment from baseline BCVA was significantly greater in both DEXimplant treatment groups than in the sham group from day 30 to day 90 (P⬍0.001; with the greatest response (29%) at day60. In the DEX implant 0.7-mg group, the proportion of eyes Visual Acuity. Eyes receiving DEX implant 0.7 or 0.35 mg
achieving at least a 15-letter improvement at day 180 was 22% achieved a 15-letter improvement in BCVA significantly faster (92/427), but this was not significantly different from the sham than the eyes receiving sham treatment. This is seen in the cumu- group (18%; 75/426). There were no statistically significant dif- lative response rate curves for the time to reach a 15-letter im- ferences between the DEX implant 0.7-mg and 0.35-mg treatment provement from baseline BCVA (primary outcome measure; groups at any follow-up visit. Similar results for this analysis were P⬍0.001). The response rates in the DEX implant 0.7-mg group seen when the 2 phase III studies were analyzed separately. In were often numerically higher than those in the DEX implant addition, in an analysis of the day-180 results that excluded those 0.35-mg group, but this difference was not statistically significant.
patients whose last visit was later than day 180, the difference At day 180, the cumulative response rate was 41% in the DEX between the DEX implant 0.7-mg group (55/208; 26.4%) and the implant 0.7-mg group, 40% in the DEX implant 0.35-mg group, sham group (39/229; 17.0%) was statistically significant at day 180 and 23% in the sham group. Between-group differences in the time (P ⫽ 0.017; to a 15-letter gain were also statistically significant when each of When each of the phase III studies was evaluated separately, the 2 phase III studies was analyzed separately available the first study did not meet its regulatory primary end point Volume 117, Number 6, June 2010 Table 1. Demographic and Baseline Characteristics DEX Implant 0.35 mg
DEX Implant 0.7 mg (n ⴝ 427)
(n ⴝ 414)
Sham (n ⴝ 426)
Asian (excluding Japanese) Diagnosis in study eye Duration of macular edema Mean duration (range) Mean baseline visual acuity, letters ⫾ SD 54.3⫾9.93 (20/80) 53.9⫾10.41 (20/80) 54.8⫾9.86 (20/80) (Snellen equivalent) Mean baseline retinal thickness Prior laser photocoagulation Other procedures for RVO Intraocular injection Diabetes mellitus Coronary artery disease IOP-lowering medication use at baseline BRVO ⫽ branch retinal vein occlusion; CRVO ⫽ central retinal vein occlusion; DEX implant ⫽ dexamethasone intravitreal implant (OZURDEX,Allergan Inc., Irvine, CA); IOP ⫽ intraocular pressure; NS ⫽ not significant; RVO ⫽ retinal vein occlusion; SD ⫽ standard deviation.
*P values were based on analysis of variance for age and the Pearson chi-square test for other variables.
†Caucasian versus non-Caucasian.
‡Based on biomicroscopic data at baseline.
(proportion of eyes achieving at least 15 letters of improvement implant 0.35-mg group and the sham group was no longer statis- from baseline BCVA) at day 180 (P ⫽ 0.087 for DEX implant 0.7 tically significant. Throughout the study, eyes treated with DEX mg vs. sham), although the difference between the DEX implant implant were less likely than sham-treated eyes to experience a 0.7-mg group and sham groups was statistically significant on days decrease in vision of ⱖ15 letters 30 to 90 (Pⱕ0.039).The second study did meet its primary end The mean increase from baseline visual acuity was significantly point (time to 15-letter gain; P⬍0.001 for DEX implant 0.7 mg vs.
greater in both DEX implant treatment groups than in the sham group from day 30 to day 180 (Pⱕ0.006; with the greatest The proportion of eyes achieving at least 10, 11, 12, 13, or 14 between-group difference (⬃7 letters) at day 60. There were no letters of improvement from baseline BCVA was signif- statistically significant differences between the DEX implant icantly greater in both DEX implant groups than in the sham group 0.7-mg and 0.35-mg treatment groups at any follow-up visit.
at days 30, 60, and 90 (P⬍0.001). At day 180, the percentage of Retinal Thickness. The mean decrease in central subfield
eyes achieving at least 10, 12, 13, or 14 letters of improvement was retinal thickness was significantly greater with DEX implant 0.7 still significantly greater in the DEX implant 0.7-mg group than in mg (208⫾201 m) and 0.35 mg (177⫾197 m) than with sham the sham group (Pⱕ0.040), but the difference between the DEX treatment (85⫾173 m; P⬍0.001) at day 90 but not at day 180 Haller et al 䡠 Novel Dexamethasone Drug Delivery System in Treatment of RVO Figure 2. Time to achieve 15 letters of improvement from baseline BCVA. BCVA ⫽ best corrected visual acuity; DEX ⫽ dexamethasone intravitreal
implant.
. The number of eyes with retinal thickness ⱕ250 m in the sham group were greater with shorter duration of ME, but at day 90 was 141 of 389 (36.3%) in the DEX implant 0.7-mg the response to treatment was not.
group, 131 of 384 (34.1%) in the DEX implant 0.35-mg group,and 62 of 397 (15.6%) in the sham group (P⬍0.001, among Subgroup Analysis by Baseline Retinal Vein Occlusion Di-
The overall incidence of ocular adverse events was significantly agnosis. The key efficacy analyses (time to 15-letter improve-
higher in the DEX implant 0.7-mg group (62.9%) and DEX ment, proportion of eyes achieving at least a 15-letter improve- implant 0.35-mg group (61.9%) than in the sham group (42.8%; ment, and mean change from baseline BCVA) were evaluated for P⬍0.001). Ocular adverse events in the study eye reported by the BRVO and CRVO populations separately (prospectively de- more than 2% of patients in any treatment group or for which there fined subgroup analysis). In general, the response to treatment in was a statistically significant difference between a DEX implant both subgroups was qualitatively similar to the responses seen in group and sham are listed in The only adverse events that the overall population, but the response in the sham group was occurred significantly more frequently in either DEX implant greater in the BRVO subgroup than in the CRVO subgroup in all treatment group than in the sham group were eye pain (P ⫽ 0.023), efficacy analyses The difference between the sham groups ocular hypertension (Pⱕ0.002), and anterior chamber cells was particularly marked in the analysis of mean change from (Pⱕ0.031). The incidence of retinal neovascularization was sig- baseline BCVA Mean BCVA slowly improved over the nificantly lower in the DEX implant 0.7-mg group than the sham course of the study among BRVO eyes treated with sham, but group (P ⫽ 0.032). There was no statistically significant difference gradually declined to below baseline levels among CRVO eyes in the ocular adverse event incidence between the DEX implant treated with sham.
0.7-mg and 0.35-mg groups.
Subgroup Analysis by Duration of Macular Edema at Base-
Total cataract adverse events during the study (including cor- line. A post hoc subgroup analysis based on the duration of ME at
tical, nuclear, and subcapsular) were reported in the study eye for baseline found that the response to treatment was often greater 7.3% of patients (31/423) in the DEX implant 0.7-mg group, 4.1% among eyes with a shorter duration of ME at baseline (ⱕ90 days) of patients (17/411) in the DEX implant 0.35-mg group, and 4.5% compared with a longer duration of ME. For example, at day 60 of patients (19/422) in the sham group (P ⫽ 0.079). For 21 of these (peak response) in the DEX implant 0.7-mg group, the proportion 67 patients, the cataract adverse events were bilateral. The number of eyes improving by ⱖ15 letters was 38% in eyes with ME of cataracts that were subcapsular was 7 of 31 in the DEX implant duration ⱕ90 days and 27% in eyes with ME duration ⬎90 days; 0.7-mg group, 4 of 17 in the DEX implant 0.35-mg group, and 3 in the DEX implant 0.35-mg group, the proportion of eyes im- of 19 in the sham group (P ⫽ 0.443). Three patients (1 in each proving ⱖ15 letters was 35% in eyes with ME duration ⱕ90 days group) had cataract extraction during the study.
and 27% in eyes with ME duration ⬎90 days. Similarly, the peak Two patients had retinal detachments in the study eye: 1 in the mean change from baseline BCVA (day 60) in the DEX implant sham group and 1 in the DEX implant 0.7-mg group; none oc- 0.7-mg group was 11.7 letters in eyes with ME duration ⱕ90 days curred in the nonstudy eye. There was no statistically significant and 9.4 letters in eyes with ME duration ⬎90 days; in the DEX difference between the treatment groups in the incidence of vitre- implant 0.35-mg group, the peak mean change was 9.9 letters in ous or retinal hemorrhage. No cases of endophthalmitis were eyes with ME duration ⱕ90 days and 9.6 letters in eyes with ME duration ⬎90 days. Improvements in the sham group were also Ocular hypertension was reported as an adverse event in sig- greater among patients with shorter duration of ME. Greater im- nificantly more eyes in both DEX implant groups than in the sham provements in BCVA with shorter ME duration were also seen in group (Pⱕ0.002; Changes in IOP in the DEX implant the BRVO subgroup. In eyes with CRVO, however, improvements groups peaked at day 60 and were no different from sham by day Volume 117, Number 6, June 2010 eye in each of the DEX implant groups had its procedure forneovascular glaucoma rather than treatment-related increased IOP.
The percentage of eyes receiving IOP-lowering medication in-creased in the DEX implant treatment groups from approximately6% at the beginning of the study to approximately 24% by day180; there was no change in the sham group.
The overall rate of nonocular adverse events was similar in the 3 treatment groups (DEX implant 0.7 mg: 830%; DEX implant0.35 mg: 29%; sham: 31%). There were no statistically significantamong-group differences at baseline or in the change from base-line in any vital signs or physical findings.
Serious Adverse Events. The overall incidence of serious
adverse events was 5.0% (21/421) in the DEX implant 0.7-mggroup, 6.6% (27/412) in the DEX implant 0.35-mg group, and5.9% (25/423) in the sham group (no statistically significantbetween-group difference). One additional patient in the shamgroup developed a recurrence of melanoma in the right axilla,which met the criteria for a serious event but was reported asnonserious by the investigator.
A single treatment with DEX implant 0.7 or 0.35 mg pro-duced significantly greater improvements in visual acuitythan did a sham procedure in eyes with vision loss due toME associated with BRVO or CRVO. This was evident inthe results for several efficacy measures, including the timeto achieve a 15-letter improvement from baseline BCVA(primary outcome measure for the 2 pooled studies), theproportion of eyes achieving at least a 15-letter improve-ment, the proportion of eyes with BCVA worsening of atleast 15 letters, and mean change from baseline BCVA. The Figure 4. Percentage of eyes achieving at least 15 letters of improvement
greater improvements in visual acuity with DEX implant from baseline BCVA. P values are for DEX implant 0.7 or 0.35 mg versus were accompanied by greater decreases in OCT-measured sham. A, Percentage of eyes achieving at least 15 letters of improvement
central retinal thickness than were seen with sham treat- at each study visit. B, Percentage of eyes achieving at least 15 letters of
ment. Although the improvements in various efficacy mea- improvement at day 180. Showing the data for all patients and the data sures trended higher in eyes receiving DEX implant 0.7 mg after excluding patients who returned for the day 180 visit after day 180(per protocol analysis). BCVA ⫽ best-corrected visual acuity; DEX im- compared with DEX implant 0.35 mg, the differences were plant ⫽ dexamethasone intravitreal implant.
not statistically significant.
A statistically significant difference between the sham group and both DEX implant groups was seen as early as 180 Most eyes with increases in IOP were successfullymanaged with topical IOP-lowering medication, but 5 eyes (3 in the first efficacy visit at day 30 and often persisted until day the DEX implant 0.7-mg group, 2 in the DEX implant 0.35-mg 180 in the DEX implant 0.7-mg group. In the primary group) required a procedure to reduce IOP (e.g., trabeculoplasty, efficacy measure of time to at least 15-letter gain, the tube insertion, deep sclerectomy, or cyclocryotherapy). Of these, 1 cumulative response rate curves for the DEX implant treat- Table 2. Percentage of Eyes Achieving Improvements from Baseline Best-Corrected Visual Acuity of 10 to 14 Letters Percentage of Eyes/P Value vs. Sham
DEX Implant Dose
ⱖ10 letters increase ⱖ11 letters increase ⱖ12 letters increase ⱖ13 letters increase ⱖ14 letters increase DEX implant ⫽ dexamethasone intravitreal implant; NS ⫽ not statistically significant (Pⱖ0.05).
P values are listed for the comparison of DEX implant versus sham for each dosing group at each follow-up visit.
Shading indicates differences that reached statistical significance.
Haller et al 䡠 Novel Dexamethasone Drug Delivery System in Treatment of RVO this time point. This suggests that factors in addition tochanges in central retinal thickness may be affecting visualacuity in RVO eyes with ME treated with DEX implant.
A possible criticism of this study is that it included patients with BRVO and patients with CRVO. This studywas aimed at evaluating the response to treatment with DEXimplant in eyes with ME due to BRVO or CRVO. AlthoughBRVO and CRVO are arguably different disease entities(e.g., differing in natural history and the sites of occlusion),they share numerous characteristics, including risk factors,and there is no compelling evidence that the ME resultingfrom the occlusive events in either BRVO or CRVO differssubstantively in terms of pathophysiology. Nonetheless, al-though patients with BRVO and patients with CRVO wereincluded in this study, a prospectively defined subgroup Figure 5. Percentage of eyes experiencing at least a 15-letter worsening
analysis based on baseline diagnoses (BRVO/CRVO) was from baseline BCVA. BCVA ⫽ best corrected visual acuity; DEX implant included in the protocol to address concerns that one group ⫽ dexamethasone intravitreal implant.
might have a differential response that caused the benefits ofthe drug therapy on the other group to be either under- oroverestimated. The results of this analysis confirm previous ment groups separated from the curve for the sham group as observations that CRVO is a more visually disabling disor- early as day 30 P⬍0.001). A statistically significant der than BRVO. Eyes with CRVO did not respond as well difference between the sham group and both DEX implant to therapy as eyes with BRVO, and they were less likely to groups was also seen as early as day 30 in the proportion of improve without therapy. The percentage of eyes in the eyes with at least a 15-letter improvement. The proportion DEX implant groups achieving at least a 15-letter improve- of eyes with at least a 15-letter improvement peaked at day ment from baseline BCVA was sustained at a slightly higher 60 and was maintained through day 90 but there rate for longer among eyes with BRVO than eyes with was no statistically significant difference between either CRVO. In addition, in the sham groups mean BCVA DEX implant group and the sham group at day 180 gradually improved with time in the BRVO eyes, whereas Of potential significance is the fact that approximately mean BCVA steadily declined with time in the CRVO half of all patients (614/1267) had their day-180 study visit considerably later than day 180. Because the DEX implant A potentially important finding of a post hoc analysis in was designed to deliver therapeutic levels of dexamethasone this study was that shorter ME duration at baseline was for only 6 months, these patients may have had subthera- associated with greater improvements in BCVA after DEX peutic drug levels at the time of their last study visit. When implant. A similar effect of ME duration was seen in the these patients were excluded from the analysis, the differ- Standard Care vs. Corticosteroid for RVO Study (SCORE ence between the DEX implant 0.7-mg group and the sham group was statistically significant at day 180 (P ⫽ 0.017) The adverse events that occurred at a significantly higher rate in the DEX implant treatment group than in the sham Eyes treated with DEX implant were less likely to expe- group were eye pain, ocular hypertension, and anterior rience a 15-letter decrease in BCVA than were eyes receiv-ing sham treatment this difference remained statis-tically significant through day 180. This finding providesfurther information on the natural history of RVO by con-firming that significant numbers of patients with untreatedRVO (particularly those with CRVO; will continueto lose visual acuity over time. It also demonstrates thattreatment with DEX implant can both reduce the risk offurther vision loss and increase the chance of improvementsin visual acuity.
Both DEX implant 0.7 mg and 0.35 mg produced sig- nificantly greater mean improvements from baseline BCVAthan did sham treatment (Pⱕ0.006) throughout the studyperiod.
The improvements in visual acuity outcomes persisted longer than the reduction in retinal thickness as measured byOCT. Although there were statistically significant between-group differences favoring DEX implant 0.7 mg over sham Figure 6. Mean change from baseline BCVA. P values are for DEX
in several visual acuity measures at day 180, there was no implant 0.7 mg versus sham. BCVA ⫽ best-corrected visual acuity; DEX between-group difference in change in retinal thickness at implant ⫽ dexamethasone intravitreal implant.
Volume 117, Number 6, June 2010 Table 3. Mean Change in Central Retinal Thickness DEX Implant 0.7 mg
DEX Implant 0.35 mg
P Value vs. Sham
(n ⴝ 427)
(n ⴝ 414)
(n ⴝ 426)
0.35 mg/0.7 mg
All eyes (m⫾SD) All eyes (m⫾SD) DEX implant ⫽ dexamethasone intravitreal implant; NS ⫽ not statistically significant (Pⱖ0.05); SD ⫽ standarddeviation.
chamber cells these are all adverse events that than in the sham group (P ⫽ 0.032). This finding, combined have been associated with intravitreal injection or cortico- with the lower rate of visual loss in the active treatment steroid There was no statistically sig- groups, raises the possibility that DEX implant may have an nificant difference between the treatment groups in the impact on ischemia and disease progression, as well as incidence of vitreous or retinal hemorrhage, and no cases of increasing the chance of improvements in visual acuity.
endophthalmitis were reported.
Randomized, controlled clinical trials have also demon- Results from a previous phase 2 trial of DEX implant in strated the efficacy of laser the cortico- patients with persistent ME found a lower rate of both steroid triamcinolone and the anti-VEGF cataract and IOP increases than has been reported with other therapy in the treatment of ME secondary to corticosteroids (including triamcinolone RVO. In the SCORE study, grid photocoagulation and There was no statistically significant difference in the rate of repeated injections of triamcinolone acetonide 1 or 4 mg cataract among the treatment groups in the present study, seemed to be equally effective in producing improvements but 180 days may not be a long enough study period to in BCVA in patients with ME due to BRVO: a 15-letter gain detect an effect of treatment on cataract formation. The in BCVA from baseline to month 12 in 29% of patients majority of eyes treated with DEX implant did not experi- treated with grid photocoagulation and 26% and 27% of ence a substantial increase in IOP. At peak (day 60), less patients treated with triamcinolone acetonide 1 or 4 mg, than 16% of all eyes had an increase in IOP to ⱖ25 mmHg.
Rates of elevated IOP and cataract were Increases in IOP were typically transient, and there was no similar for the grid laser and 1-mg triamcinolone groups, but difference between the DEX implant groups and the sham higher in the 4-mg triamcinolone group; 41% of patients group by day 180 Nearly all cases of elevated IOP treated with triamcinolone acetonide 4 mg initiated IOP- were managed with observation or topical medications lowering medication during the 12-month In a alone. Notably, the incidence of retinal neovascularization separate report of the SCORE study regarding eyes with ME was significantly lower in the DEX implant 0.7-mg group due to CRVO, triamcinolone acetonide 1 mg and 4 mg were Table 4. Ocular Adverse Events in the Study Eye Reported for ⬎2% of Patients in any Treatment Group or for Which There Was a Statistically Significant Difference between a DEX Implant Group DEX Implant 0.7 mg
DEX Implant 0.35 mg
P Value vs. Sham
(n ⴝ 421)
(n ⴝ 412)
(n ⴝ 423)
0.35 mg/0.7 mg
Conjunctival hemorrhage Conjunctival hyperemia Ocular hypertension Vitreous floaters Vitreous detachment Retinal hemorrhage Foreign-body sensation Vitreous hemorrhage Conjunctival edema Visual acuity reduced Anterior chamber cells DEX implant ⫽ dexamethasone intravitreal implant; NS ⫽ not statistically significant (Pⱖ0.05).
*Includes adverse events listed as cataract, cataract cortical, cataract nuclear, or cataract subcapsular.
Haller et al 䡠 Novel Dexamethasone Drug Delivery System in Treatment of RVO Figure 7. Efficacy comparison for BRVO and CRVO subgroups. A, Time to achieve 15 letters of improvement from baseline. B, Percentage of eyes
achieving 15 letters of improvement from baseline BCVA. C, Mean change from baseline BCVA. BCVA ⫽ best-corrected visual acuity. BRVO ⫽ branch
retinal vein occlusion; CRVO ⫽ central retinal vein occlusion; DEX implant: dexamethasone intravitreal implant.
shown to produce a 15-letter gain in BCVA from baseline to trials of ranibizumab (BRAVO and CRUISE studies; pre- month 12 in 27% and 26%, respectively. The rates of cataract sented at Retina Congress ranibizumab 0.5 mg and elevated IOP were similar for the observation and 1-mg injected monthly produced a 15-letter gain in BCVA from groups, but higher in the 4-mg group; 35% of patients with baseline to 6 months in 61% of patients with BRVO and 48% CRVO treated with triamcinolone acetonide 4 mg initiated of patients with CRVO. In the absence of well-controlled IOP-lowering medication during the 12-month In 2 clinical trials that directly compare these therapeutic ap- recently completed, but as yet unpublished, 6-month clinical proaches with dexamethasone, it is difficult to make mean- Volume 117, Number 6, June 2010 als. For example, in the BRAVO, CRUISE, and SCOREstudies, eyes had ME of short duration relative to the DEXimplant studies. In the SCORE study, 37% to 44% ofpatients had a duration of ME of less than 3 compared with 14% to 17% of patients in the present study.
Post hoc analyses of both the present study and the SCOREstudy data suggest that patients with shorter duration of MEmay have a greater response to The studiesalso had different inclusion criteria and visit schedules,among other protocol differences that may have affectedsome study outcomes.
As has been seen with other medical therapies for RVO, the response to treatment with DEX implant is of limitedduration, lasting between 90 and 180 days depending on thepatient population and outcome measure. Further study isneeded to determine the response to repeated treatments andthe optimum retreatment interval. A long-term study ofrepeated treatment has recently been completed and is beingprepared for publication.
A potential limitation of the present study was the use of sham treatment rather than laser treatment as the controlgroup. Sham treatment was chosen on the basis of the phase2 trial data and because this study included patients withBRVO and CRVO in a single trial, and there is evidencesuggesting that laser photocoagulation can improve visionin eyes with ME associated with BRVO, but notMoreover, 33% to 50% of eyes with BRVOmay regain vision of ⱖ20/40 within 6 months, and 70%may gain ⱖ2 lines of vision in 12 months without anytreatment at Approximately 10% of cases of nonisch-emic CRVO may also resolve completely without treatment(although most eyes continue to lose vision, and the naturalhistory of the disease is generally Conse-quently, it can be difficult to differentiate the benefits of anyintervention from the natural course of the disease withoutan untreated control group.
Another potential limitation of the study was that pa- tients with CRVO were not screened for perfused or isch-emic disease. The relatively good vision (⬎20/200) of thepatient population at baseline suggests that most patientshad perfused disease, but the development of neovascular-ization in 2.6% of sham patients suggests that at least somepatients had ischemic disease. The presence of some pa-tients with ischemic disease might have caused the benefitsof treatment on the larger population of patients with per-fused disease to be slightly underestimated. Moreover, de-spite the apparent inclusion of some patients with ischemicCRVO, no conclusions should be drawn from the presentstudy regarding the effectiveness of DEX implant in isch- Figure 8. Percentage of eyes with notable changes in IOP. A, Percentage
emic patients.
of eyes with IOP of at least 35 mmHg. B, Percentage of eyes with IOP of
In conclusion, the results of the present study demonstrate at least 25 mmHg. C, Percentage of eyes with an increase in IOP of at least
that DEX implant can both reduce the risk of further vision loss 10 mmHg. DEX implant ⫽ dexamethasone intravitreal implant; IOP ⫽ and increase the chance of improvement in visual acuity in eyes with BRVO or CRVO. The results of this study furtherdemonstrate that, when eyes with RVO are left untreated, asignificant percentage will either fail to improve or experience ingful statements about the relative merits of these different further loss of visual acuity. The DEX implant was well treatments. This difficulty is compounded by the differences tolerated, producing generally transient, moderate, and readily between the patient populations and the study designs of the managed increases in IOP in less than 16% of eyes. Overall, DEX implant, triamcinolone, and ranibizumab clinical tri- this study suggests that DEX implant could be a valuable new Haller et al 䡠 Novel Dexamethasone Drug Delivery System in Treatment of RVO treatment option for eyes with visual loss due to ME associated vs Corticosteroid for Retinal Vein Occlusion (SCORE) Study with BRVO or CRVO.
report 5. Arch Ophthalmol 2009;127:1101–14.
Acknowledgments. The writing assistance and manuscript
14. Ramezani A, Entezari M, Moradian S, et al. Intravitreal tri- preparation services of Amy Lindsay, PhD (Lindsay Biomedical amcinolone for acute central retinal vein occlusion: a random- Communications, Inc.), were retained by Allergan, Inc., for the ized clinical trial. Graefes Arch Clin Exp Ophthalmol 2006; writing committee. Dr. Lindsay, who did not meet authorship 244:1601– 6.
criteria, attended all meetings and conference calls of the writing 15. Kuppermann BD, Blumenkranz MS, Haller JA, et al, Dexa- committee; collated committee drafts, comments, and references; methasone DDS Phase II Study Group. Randomized con- compiled an outline for discussion and review; drafted a first trolled study of an intravitreous dexamethasone drug delivery manuscript iteration on the basis of committee discussion and system in patients with persistent macular edema. Arch Oph- reworking of the detailed outline; and subsequently compiled the thalmol 2007;125:309 –17.
editorial comments of the group and executed several rounds of 16. Antonetti DA, Wolpert EB, DeMaio L, et al. Hydrocortisone revisions under writing committee guidance.
decreases retinal endothelial cell water and solute flux coin-cident with increased content and decreased phosphorylationof occludin. J Neurochem 2002;80:667–77.
17. Leopold IH. Nonsteroidal and steroidal anti-inflammatory agents. In: Sears ML, Tarkkanen A, eds. Surgical Pharmacol-ogy of the Eye. New York: Raven Press, 1985:83–133.
1. Yau JW, Lee P, Wong TY, et al. Retinal vein occlusion: an 18. Nauck M, Karakiulakis G, Perruchoud AP, et al. Corticoste- approach to diagnosis, systemic risk factors and management.
roids inhibit the expression of the vascular endothelial growth Intern Med J 2008;38:904 –10.
factor gene in human vascular smooth muscle cells. Eur 2. Mitchell P, Smith W, Chang A. Prevalence and associations of J Pharmacol 1998;341:309 –15.
retinal vein occlusion in Australia: the Blue Mountains Eye 19. Tennant JL. Cystoid maculopathy: 125 prostaglandins in oph- Study. Arch Ophthalmol 1996;114:1243–7.
thalmology. In: Emery JM, ed. Current Concepts in Cataract 3. Royal College of Ophthalmologists. Retinal vein occlusion Surgery: Selected Proceedings of the Fifth Biennial Cataract (RVO) interim guidelines. February 2009. Available at: Surgical Congress. Section 3. St. Louis, MO: Mosby; 1978: Accessed January 6, 2009.
20. Ip MS, Gottlieb JL, Kahana A, et al. Intravitreal triamcin- 4. Klein R, Klein BE, Moss SE, Meuer SM. The epidemiology of olone for the treatment of macular edema associated with retinal vein occlusion: the Beaver Dam Eye Study. Trans Am central retinal vein occlusion. Arch Ophthalmol 2004;122: Ophthalmol Soc 2000;98:133– 43.
5. Central Vein Occlusion Study Group. Natural history and 21. Williamson TH, O'Donnell A. Intravitreal triamcinolone ace- clinical management of central retinal vein occlusion. Arch tonide for cystoid macular edema in nonischemic central ret- Ophthalmol 1997;115:486 –91.
inal vein occlusion. Am J Ophthalmol 2005;139:860 – 6.
6. Medscape. Fonrose M. Retinal vein occlusion. Emedicine.
22. Krepler K, Ergun E, Sacu S, et al. Intravitreal triamcinolone acetonide in patients with macular oedema due to central Accessed January 6, 2010.
retinal vein occlusion. Acta Ophthalmol Scand 2005;83: 7. University of Iowa Health Care. Hayreh SS. Central retinal vein occlusion. March 2003. Available at: 23. Jonas JB, Akkoyun I, Kamppeter B, et al. Intravitreal triam- Accessed January 6, 2010.
cinolone acetonide for treatment of central retinal vein occlu- 8. Rehak J, Rehak M. Branch retinal vein occlusion: pathogen- sion. Eur J Ophthalmol 2005;15:751– 8.
esis, visual prognosis, and treatment modalities. Curr Eye Res 24. Jonas JB, Akkoyun I, Kamppeter B, et al. Branch retinal vein occlusion treated by intravitreal triamcinolone acetonide. Eye 9. Antonetti DA, Barber AJ, Khin S, et al, Penn State Retina Research Group. Vascular permeability in experimental dia- 25. Cekic O, Chang S, Tseng JJ, et al. Intravitreal triamcinolone betes is associated with reduced endothelial occludin content: treatment for macular edema associated with central retinal vascular endothelial growth factor decreases occludin in reti- vein occlusion and hemiretinal vein occlusion. Retina 2005; nal endothelial cells. Diabetes 1998;47:1953–9.
25:846 –50.
10. Campochiaro PA, Hafiz G, Shah SM, et al. Ranibizumab for 26. Chen SD, Sundaram V, Lochhead J, Patel CK. Intravitreal macular edema due to retinal vein occlusions: implication of triamcinolone for the treatment of ischemic macular edema VEGF as a critical stimulator. Mol Ther 2008;16:791–9.
associated with branch retinal vein occlusion. Am J Ophthal- 11. SCORE Study Research Group. A randomized trial comparing mol 2006;141:876 – 83.
the efficacy and safety of intravitreal triamcinolone with stan- 27. Cheng KC, Wu WC. Intravitreal triamcinolone acetonide for dard care to treat vision loss associated with macular edema patients with macular edema due to branch retinal vein occlu- secondary to branch retinal vein occlusion: the Standard Care sion. Kaohsiung J Med Sci 2006;22:321–30.
vs Corticosteroid for Retinal Vein Occlusion (SCORE) Study 28. Oh JY, Seo JH, Ahn JK, et al. Early versus late intravitreal report 6. Arch Ophthalmol 2009;127:1115–28.
triamcinolone acetonide for macular edema associated with 12. Two phase III studies of Lucentis show early and sustained improve- branch retinal vein occlusion. Korean J Ophthalmol 2007;21: ment in vision in patients with retinal vein occlusion [press release].
Medical News Today. October 2009. Available at: 29. Nehme A, Lobenhofer EK, Stamer WD, Edelman JL. Glu- Accessed January 6, cocorticoids with different chemical structures but similar glucocorticoid receptor potency regulate subsets of common 13. SCORE Study Research Group. A randomized trial comparing and unique genes in human trabecular meshwork cells. BMC the efficacy and safety of intravitreal triamcinolone with ob- Med Genomics [serial online] 2009;2:58. Available at: servation to treat vision loss associated with macular edema Accessed March 5, secondary to central retinal vein occlusion: the Standard Care Volume 117, Number 6, June 2010 30. Early Treatment Diabetic Retinopathy Study design and base- 34. McIntosh RL, Mohamed Q, Saw SM, Wong TY. Interventions line patient characteristics. ETDRS report number 7. Ophthal- for branch retinal vein occlusion: an evidence-based system- atic review. Ophthalmology 2007;114:835– 46.
31. Ramchandran RS, Fekrat S, Stinnett SS, Jaffe GJ. Fluocin- 35. A randomized clinical trial of early panretinal photocoagula- olone acetonide sustained drug delivery device for chronic tion for ischemic central vein occlusion: the Central Vein central retinal vein occlusion: 12-month results. Am J Oph- Occlusion Study Group N report. Ophthalmology 1995;102: 1434 – 44.
32. Cekic O, Chang S, Tseng JJ, et al. Intravitreal triamcinolone 36. Branch Vein Occlusion Study Group. Argon laser photocoag- injection for treatment of macular edema secondary to branch ulation for macular edema in branch vein occlusion. Am J retinal vein occlusion. Retina 2005;25:851–5.
Ophthalmol 1984;98:271– 82.
33. Mohamed Q, McIntosh RL, Saw SM, Wong TY. Interventions 37. Finkelstein D. Ischemic macular edema: recognition and fa- for central retinal vein occlusion: an evidence-based system- vorable natural history in branch vein occlusion. Arch Oph- atic review. Ophthalmology 2007;114:507–17.
Footnotes and Financial Disclosures
Originally received: November 18, 2009.
This article contains online-only material. The following should appear Final revision: March 10, 2010.
online only: and The GENEVA Study Group.
Accepted: March 11, 2010.
Financial Disclosure(s): Available online: April 22, 2010.
Manuscript no. 2009-1609.
The author(s) have made the following disclosure(s): Dr. Haller is a 1 Wills Eye Institute, Philadelphia, Pennsylvania.
consultant with Genentech and Allergan and an equity owner with Macu- sight and OptiMedica. Dr. Bandello is a consultant and lecturer with Ospedale Maggiore-Clinica Oculistica, Udine, Italy.
Allergan and Novartis. Dr. Belfort is a consultant and lecturer with Aller- 3 Vision Institute, Federal University of São Paulo, Brazil.
gan and Alcon. Dr. Blumenkranz is a consultant with Allergan, Macusight, 4 Stanford University, Stanford, California.
and Genentech, an equity owner with Macusight and Optimedica, a lecturer with Allergan, and a patent holder with Optimedica. Dr. Gillies is a University of Sydney, Sydney, Australia.
consultant with Allergan, Novartis, and Pfizer. Dr. Heier is a consultant for 6 Ophthalmic Consultants of Boston, Boston, Massachusetts.
and received financial support from Alimera, Allergan, Genetech, and 7 Tel-Aviv Medical Center, Tel-Aviv, Israel.
Regeneron, and is a lecturer for Regeneron. Dr. Lowenstein is a consultant for Allergan and Notal Vision and a lecturer for Novartis and Alcon. Dr.
Asan Medical Center, Seoul, South Korea.
Yoon is a consultant for Allergan and a lecturer for Alcon. Drs. Jacques, 9 Allergan, Inc., Irvine, California, at the time of study.
Jiao, Li, and Whitcup are employees of Allergan, Inc.
10 Allergan, Inc., Irvine, California.
Sponsored by Allergan, Inc., which participated in the design of the study, *GENEVA: Global Evaluation of implaNtable dExamethasone in retinal data analysis, and interpretation, and supervised the preparation of the Vein occlusion with macular edemA. A list of the members of the manuscript and approved the final version.
GENEVA study group is available at A preliminary report of this study was presented at: Retina Congress 2009, Julia A. Haller, MD, Wills Eye Institute, 840 Walnut St., Suite 1510, September 30 to October 4, 2009, New York.
Philadelphia, PA 19107. E-mail: Haller et al 䡠 Novel Dexamethasone Drug Delivery System in Treatment of RVO Appendix 1. The GENEVA Study Group
Shabrawi, Sameer Elsherbiny, Harry Engel, Nicholas En-gelbrecht, Jan Ernest, Rohan Essex, Nicole Eter, Richard Writing Committee: Francesco Bandello, Rubens Belfort, Evans, Anna Fakadej, Philip Falcone, Dorothy Fan, Joseph Mark Gillies, Julia Haller (Chair), Jeff Heier, Anat Loewen- T. Fan, Michel Eid Farah, Sam Farah, Leonard Feiner, stein, Mark Blumenkranz, Young-Hee Yoon, Joanne Li, Robert M. Feldman, Joseph Ferencz, Alvaro Fernandez Scott M. Whitcup.
Vega Sanz, João Luiz Lobo Ferreira, Joao Figueira, Mitch- The GENEVA Study Group Investigators: Thomas M.
ell Fineman, Ivan Fiser, Gary Fish, Richard H. Fish, Barron Aaberg, Prema Abraham, Suel Abujamra, James Acton, Aneta Fishburne, Stefen J. Fisher, Ross Fitzsimons, Christina Adamczyk-Ludyga, Mohamed Adenwalla, David D. Agahi- Flaxel, Emily Fletcher, Marisa Flores-Aguilar, Silvia Flo- gian, Vitor Agoas, Miguel Aguilar-Mendoza, Sabine Aisen- rez, Harry Flynn, Steven Fogarty, Antonio Folgado, Bradley brey, Suhail Alam, David Albiani, Bogdan Alexandrescu, Scott Foster, Gregory M. Fox, Donald Frambach, Carsten Mario Manuel Alfaiate, Souha Allam, Herbert Paulo Almeida, Framme, Stephen Fransen, Samantha Fraser-Bell, Albert Scott Anagnoste, Rajiv Anand, Nicholas Anderson, Andrew Frederick, William Freeman, Lars Freisberg, Eric Friedman, Antoszyk, Nerendra Armogan, Jennifer Arnold, Daniel Ash, Lee Friedman, Martin Fucik, Dwain G. Fuller, Jaime Walter G. Atlas, J. Albert Augustin, Marcos Pereira de Ávila, Gaitan, Ron Gallemore, Paul Gallogly, Jose Garcia Arumi, Carl Awh, Claudio Azzolini, Barbora Babkova, Sophie Jane Seema Garg, Sunir Garg, Bruce Garretson, Pierre Gastaud, Bakri, Michael J. Banach, Francesco Bandello, Adiel Barak, Alain Gaudric, Patricia Gawrilow, Peter L. Gehlbach, Orna Gaetano Barile, Derek Barker, Thomas Barnard, Karl Ulrich Geyer, A. Thomas Ghuman, Fabrizio Giansanti, Alberto Bartz-Schmidt, Maurizio Battaglia Parodi, Caroline Baumal, Luiz Gil, Howard D. Gilbert, Mark Gillies, Jean-Francois Petr Bedrich, Paul Beer, Rubens Belfort Mattos Junior, Lu- Girmens, Antonio Giubilato, Agnes Glacet-Bernard, David ciano Bellini, Jeffrey Benner, William Benson, Matthew Benz, Glaser, Ronald Glatzer, Debra Goldstein, André Marcelo Brian Berger, Robert Bergren, Amitabh Bharadwaj, Sathy Vieira Gomes, Hyeong Gon Yu, Fernando Pistarini Bhavan, Abdihish Bhavsar, Susanne Binder, Adriano Biondi, Gonçalves, Christine Gonzales, Joseph Googe, Lingam Go- Fiona Bishop, Norman Blair, Kevin Blinder, Mark Blumen- pal, Alan Gordon, Petrus Gous, M. Grand, Paula Cristina kranz, Andreas Bohm, Edwin E. Boldrey, Norbert Bornfeld, Grandao Magro, Manuel Granero Riano, Michael Grassi, Jesus Luigi Borrillo, David Boyer, Reagan Bradford, William James Green, Stuart Green, Zdenek Gregor, Ninel Gregori, Bridges, Luca Brigatti, Michael Briggs, H. Logan Brooks, Jr., W. Sanderson Grizzard, Carl Groenewald, Jeffrey G. Gross, David Brown, Andrew Browning, David Browning, Simon Nicole E. Gross, Allan Gruber, Gary Grutow, Ernest Guil- Brunner, Renata Brunnerova, J. Shepard Bryan, Joanna let, Anurag Gupta, Dasa Gyorgyova, Anton Haas, Karl Brydak-Godowska, Helmut Buettner, Jason Burns, Andrew Haas, Peter Hadden, Luiz Hagemann, Dean Hainsworth, F. Burrows, Brandon Busbee, Robert Butner, John Butter, Darin Haivala, Julia Haller, Lawrence Halperin, Peter Gordon Byrnes, Charleen Callahan, Peter Campochiaro, Hamer, Mark Hammer, Dennis Han, James T. Handa, Irvin Rene Alfredo Cano-Hildalgo, Tiziani Canziani, Karen Ca- Handelman, Jason Handza, Björn Harder, Simon Harding, paccioli, Antonio Capone, Trevor Carmichael, Kenneth Seenu Mavidi Hariprasad, Kristen Hartley, Paul Hartman, Carnevale, Antonio Marcelo Barbante Casella, R. Casey, Mary Elizabeth R. Hartnett, Patricia Harvey, Tarek Hassan, Antonio Castanheira-Dinis, Benito Celis, Robert Chambers, Maurice Headon, Jeffrey Heier, Libor Hejsek, Patrick Hig- Stanley Chang, Yun-Hsiang Chang, Daniel Chechik, Soon gins, Jost Hillenkamp, Allen Ho, Thomas Ho, Nancy Hole- Phaik Chee, Edwin Chen, Eric Chen, Jiann-Torng Chen, kamp, Eric Holz, Frank Holz, Philip Hooper, Janet Jill Hop- San-Ni Chen, Simon Chen, Bobby Cheng, Christophe Chi- kins, Ann Hoskin-Mott, John Hoskins, Nicholas Hrisomalos, quet, Kelvin Chong, Lawrence P. Chong, Victor Chong, Jason Hsu, Baker Hubbard III, Henry Hudson, Edward Timothy Chou, Vanessa Chow, Oldrich Chrapek, Thomas Hughes, Adrian Hunt, Alex Hunyor, Thomas Hwang, Jiunn- Chu, Jonathan Chua, Dal Chun, Hye-won Chung, Arnaldo Feng Hwang, Michael Ibarra, Nadia Incarnato, Claudia Pacheco Cialdini, Esther Ciancas, Ilona Cihelkova, Slawomir Inhetvin-Hutter, Ugo Introini, Timothy Isaacs, Niaz Islam, Cisiecki, William Clark, Tina Cleary, Rosa Coco, Marco Co- Mohan N. Iyer, Christine Jablonski, Robert L. Jack, Rama denotti, Ben Z. Cohen, Jack A. Cohen, Jeff Cohen, Brian Jager, Carolina Jahn, Cristovao Jao, Faisal Jehan, Jost Jo- Connolly, Brian Conway, Helen Cook, Blake Cooper, Lori nas, Daniel Joseph, Mandar Joshi, Bradley Jost, Bernhard Coors, Joel Corwin, José Ricardo Costa, David Cottrell, Ste- Jurklies, Ivana Kaincova, Peter Kaiser, Richard Kaiser, Bo- phen Couvillion, Jaime Craig, Alan Cruess, Gaetano Cupo, hdana Kalvodova, Bernd Kamppeter, Naresh Babu Kanann, Tim Dabbs, Sharam Danesh, Frederick Davidorf, Janet Davis, Kwon Kang, Randy S. Katz, Shalesh Kaushal, Dariusz Stefano De Cilla, Rocco De Fazio, Miguel Angel de la Fuente, Kecik, Judianne Kellaway, Kevin Kelly, Susan Kelly, Ja- Enrique Rodriguez de la Rua, Massimo De Mattia, Ahad heed Khan, Amin Kherani, Rosa Kim, Ivana Kim, Judy Deen, Lucian Del Priore, Marie-Noelle Delyfer, Christoph Kim, June-Gone Kim, Nicola Kim, Rubin Kim, Tae Wan Deuter, Dev Shanker Devadason, Robert Devenyi, David Kim, Ronald Kingsley, Richard Klein, Itamar Klemperer, d'Heurle, John Dickinson, Bernard Doft, James Dooner, Jaroslaw Kociecki, Marta Korbasova, Vladimir Korda, Deon Doubell, John Downie, Kimberly Drenser, Richard Jean-Francois Korobelnik, Zachariah Koshy, Heikki Kos- Dreyer, Yvonne D'Sousa, Ted Du, Lilianne Duarte, Harvey tamaa, Jaclyn Kovach, Igor Kozak, Vladimir Kozousek, Jan B. DuBiner, Sander Dubovy, Zora Dubska, Pravin Dugel, Krasny, Allan Kreiger, Valerie Krivosic, Joseph V. Krug, William Dunn, Jaroslava Dusova, Jan Dvorak, David Dyer, Jr., Louis Kruger, Derek Kunimoto, Baruch D. Kupper- Krzysztof Dziegielewska, Michele Earl, Catherine Egan, mann, Ron Kurtz, Aleksandra Kuznik-Borkowska, James David Eichenbaum, C. Eifrig, Anna Ells, Yosuf El- Lai, Wico Lai, Stewart Lake, Geeta Lalwani, Wai-Cheng Volume 117, Number 6, June 2010 Lam, Richard C. Lanning, Paolo Lanzetta, Wilfredo Lara, Rougier, Ricardo Infante Ribon, Renata Ricarova, Ryan Rich, Wayne I. Larrison, Rosangela Lattanzio, Adriana Lavina, Andrew Riley, Guido Ripandelli, Ekta Rishi, Kelvin Rivett, Jaco Lavinsky, Franco Lazzaroni, Eric Lee, Joo Yong Lee, Adam Rogers, Jean-Paul Romanet, Paula Jorge Rosa, Daniel Michael Lee, Sun Young Lee, Vincent Lee, Steven Leff, Rosberger, Steven Rose, Philip Rosenfeld, Robert R. Ross, John Lehr, Pauline Lenfesty, Robert Leonard, Arthur Le- Michael Rotberg, Claus Benedict Felix Roth, Daniel Roth, vine, Mark Levitan, Hilel Lewis, Simon Liew, Jennifer David Rubaltelli, Patrick Rubsamen, Alan Ruby, Jose Maria Lim, Richard Lim, Richard Lin, Peck-lin Lip, Judy Liu, Ruiz Moreno, Richard Ruiz, John Russell Gonder, Matthew Louis A. Lobes, Isaac Loose, Andrew Lotery, Claudio Russell, Jung-Wan Ryu, Helmut Sachs, Srinivas Sadda, Am- Luiz Lottenberg, Dasha Loutchkina, Da-Wen Lu, Anna mar Safar, Cecilia Salinas, Kenneth Sall, Arif Samad, Klara Lubczynska, Brandon Lujan, Anita Lyssek-Boron, Colin Samkova, Jason Sanders, Ranjit Sandhu, Sukhpal Singh Ma, Patrick Ma, David Maberley, Matthew MacCumber, KC Sandhu, Dirk Sandner, Steven R. Sanislo, Gil Sartani, Sandro Madhusudhana, Steven Madreperla, Michael Magee, Jerome Saviano, Olivier Savy, Barry A. Schechter, Howard I. Schen- Magolan, Octacílio de Oliveira Maia Junior, Andre Maia, Ajit ker, William Schiff, Frank Schlichtenbrede, Berangere Schnei- Majji, Didier Malthieu, Charles Mango, Michael Marmor, der, Lothar Schneider, Todd Schneiderman, Lisa Schocket, Luiz Marques, Daniel Martin, Jose A. Martinez, Panagiotis Ulrich Schoenherr, Dana Schoenleber, Hendrick P. Scholl, Massaoutis, Annie Mathai, Ranjana Mathur, Stefano Mattioli, Jana Schreiber, Steven D. Schwartz, Jonathan Sears, Jitka Raj K. Maturi, Iwona Mazur-Michalek, Ian McAllister, Frank Sedlakova, Christopher Seery, Clive Sell, Gaurav Shah, Mi- McCabe, Colin A. McCannel, Stewart McGimpsey, John Do- chael Shapiro, Ash Sharma, Thomas Sheidow, Shwu-Jiuan minic McHugh, Martin McKibbin, W. Copley McLean, Jr., Sheu, Tina Sheufele, Dhananjay Shukla, Joanne Siewec- Tod McMillan, Rodrigo Meireles, Carlos Sérgio Nascimento Proscinska, Eduardo Rufino Silva, Michael Singer, Shaun de Melo, Ugo Menchini, Travis Meredith, Pauline Merrill, Singer, Lawrence J. Singerman, Mandeep Singh, Yun Ching Umar Mian, Mark Michels, Edoardo Midena, William Fran- Siow, Jack O. Sipperley, Sobha Sivaprasad, Raymond Sjaarda, cis Mieler, Luca Migliavacca, David Miller, James Miller, William Snyder, Lucia Sobrin, Andrea Sodi, Sharon Solomon, Gregory Mincey, Paul Mitchell, Sandra Katsuki Mizubuti, Peter Sonkin, Gisèle Soubrane, Petr Soucek, Ben Spirn, Sunil Shaheeda Mohamed, Musadik Mohammed, Nader Moinfar, Srivastava, Kevin Stannard, Giovanni Staurenghi, Robert Joseph Moisseiev, Jordi Mones, Rodrigo Montemayor- Steinmetz, Kimberly Stepien, Walter Stern, O. Dara Steven- Lobo, Javier Montero, Nilva Imeren Bueno de Moraes, son, Donald Stewart, Jay Stewart, Ulrike Stolba, Glenn Stoller, Carlos Augusto Moreira Jr., Michael Morely, Javier Montero Cameron Stone, J. Timothy Stout, Gavin Stringfellow, Jan Moreno, Jorge Torres Moron, Victoria L. Morrison, Lawrence Studnicka, Marta Suarez-Figueroa, Jennifer Sung, Antoine Morse, Andrew Moshfeghi, Darius Moshfeghi, Cristina Muc- Susini, Royce Syracuse, Jerzy Szaflik, Mosche Szlechter, Ho- cioli, Vineeta Munshi, Raghu C. Murthy, Thet Naing, Ram- mayoun Tabandeh, Ramin Tadayoni, Walter Yukihiko Taka- achandran Nair, Joao Nascimento, Vinícius Paganini Nasci- hashi, Alexander Chater Taleb, Stephen James Talks, Luis mento, Zofia Nawrocka, Jerzy Nawrocki, Charles Newell, Tamayo, Michael Tan, Barry Taney, Dorota Tarnawska, Gior- Richard Newsom, Jackie Nguyen, Quan Nguyen, Randall gio Tassinari, J. Taylor, David Telander, Carla Territo, Edgar Long Nguyen, John Nichols, Deb Nilanjana, Barbara Noguchi, L. Thomas, Matthew Thomas, John T. Thompson, W. Scott Stuart Noorily, Roger Novack, Michael Novak, George Nova- Thompson, James S. Tiedeman, Trexler Topping, Michael lis, Dan O'Brien, Indre Offermann, Ana Paula Taba Oguido, Trese, Steven Truong, Chi Wah Tsang, Adanan Tufail, Rafael Kean Oh, Anna Okruszko, Tâmara Lopes de Oliveira, Scott Ufret-Vincenty, Radoslava Uhmannova, Lawrence Ulanski II, Oliver, Stephen Ong, Juan Orellana, Nicola Orzalesi, Louise Magdalena Ulinska, Juraj Urminsky, Harvey Uy, Hetal Vaish- O'Toole, Yesenia Ovando, Jeffrey Paccione, John Pach, Kirk nav, Monica Varano, Demetrios Vavvas, Beatriz Fernandez Packo, Maria Aneta Packowska, James Palmer, Helen Palmer, Vega Sanz, Amadeo Veloso, Igor Vicha, Francesco Viola, Karine Palombi, Andres Papp, Michel Paques, Augusto Para- Linda Visser, E. Vlkova, Michael Voelker, Doreen Volkert, nhos, Jr., Donald Park, Robert I. Park, Susanna Park, David Urs Vossmerbaumer, Cuong Vu, Sahana Vyas, Kenneth J.
Parke, Jose Carlos Pastor Jimeno, Sunil Patel, Sudheshna Wald, Joseph Walker, Andreas Walter, Robert Wang, Krzysz- Patra, Peter Reed Pavan, Ian Pearce, Krystyna Pecold, Mar- tof Wasiak, David R. Watt, Martin Weger, Frederick Weidman cella Pedio, Khaik Kee Peh, Lucia Pelosini, Scott Pendergast, III, Dov Weinberger, James M. Weisz, John Wells, III, Matt Bernard R. Perez, Don J. Perez-Ortiz, Stephen Perkins, Mark Wheatley, Sanjeewa Wickremasingh, Torsten Wiegand, Mark Peters, Thomas Pheasant, Jaroslaw Pilat, Elisabetta Pilotto, Wieland, David Will, George Williams, R. Geoff Williams, Jody Piltz-Seymour, Angelo Pirracchio, Ayala Pollack, Donald Wilson, Peter Ho Win, Glenn L. Wing, William Ezequiel Portella, Zuzana Pracharova, Matteo Prati, Jay Wirostko, Robert Wirthlin, Amy Lee Wong, Tien Wong, Gary Prensky, Renae Preston, Fernando Prieto, Stephan Tracey Wong, John Woo, Tsung-Tien Wu, Edward Wyle- Puls, Amish R. Purohit, Teresa Quintao, Firas Rahhal, Wa- gala, Jiong Yan, Chang-Hao Yang, Chung-May Yang, Yit heeda Rahman, Ayrton Roberto Branco Ramos, Sue Ram- Yang, Yit Chun Yang, David Yarian, Paul Yates, Sunita sey, Alka Rani, P. Kumar Rao, Emilio Rapizzi, Paul Yedavally, Jonathan Yoken, Lucy Young, Stephanie Raskauskas, Roberto Ratiglia, Ramakrishna Ratnakaram, Young, Rommel Josué Zago, Z. Zakov, Malgorzata Michael E. Rauser, Carl Regillo, Jiri Rehak, Elias Reichel, Zaras, Hernando Zegarra, Matthew Ziemianski, Ingrid Deborah A. Reid, Leos Rejmont, Marie Benedicte Renaud Zimmer-Galler, Alain Zourdani, and Catherine Zur.
Haller et al 䡠 Novel Dexamethasone Drug Delivery System in Treatment of RVO
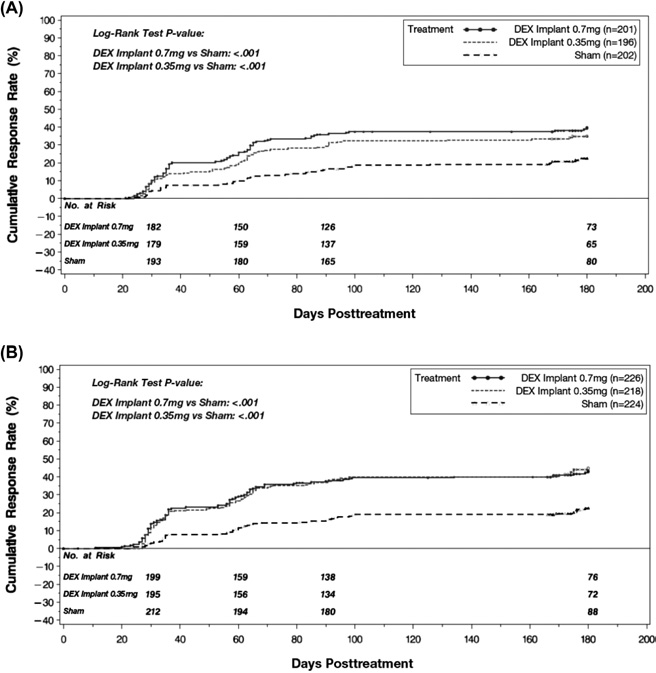
Figure 3. Time to achieve 15 letters of improvement from baseline
BCVA for the 2 phase III studies separately. A, First study. B, Second
study. BCVA ⫽ best corrected visual acuity; DEX implant ⫽ dexameth-
asone intravitreal implant.
Source: http://www.eretina.com/Paper/Ozurdex%20RVO%20study.pdf
forum-link.org
HIV and your quality of life: a guide to side effects and other complications Talking to your doctor Side effects and symptoms HIV and ageing Watch out for out-of-date information Guide to side effects and complications Section 1: General information . Introduction and changes to this edition .
Am_2004.frm.fm
Årsmelding / Annual Report 2004 Bildet på omslaget illustrerer det katastro-fale jordkskjelvet ved Sumatra den 26. desember 2004. Skjelvet forårsaket en gigantisk flodbølge (tsunami) i det Indiske hav, og førte til tap av nær 300.000 men-neskeliv. The picture on the cover illustrates the catastrophic earthquake near Sumatra on 26 December 2004. This earthquake gen-erated a huge tsunami in the Indian Ocean, claiming nearly 300,000 human lives.