Csc.hcmiu.edu.vn

VIETNAM NATIONAL UNIVERSITY – HOCHIMINH CITY
INTERNATIONAL UNIVERSITY
EFFECT OF CHOLESTEROL ON THE
PARTITIONING OF AMITRIPTYLINE INTO
LIPID MEMBRANES
A thesis submitted to
The School of Biotechnology, International University
In partial fulfillment of the requirements for the degree of
B.S. in Biotechnology
Student name: Tran Thai My Duyen – BTBTIU10019
Supervisor: Dr. Nguyen ThaoTrang
In my first word, I wish to thank my parents for their love, unconditional support and encouragement throughout my thesis. They help me realize my own potential over the years.
I would like to express my gratitude to lecturers and academic staffs in the School of Biotechnology for providing me a great working environment during the completion of my thesis work.
Next, I would like to express my deepest appreciation to my supervisor at the school of Biotechnology - International University, Dr. Nguyen Thao Trang, who gave me huge support all along. I really admire her wide knowledge and skills in scientific area. During my thesis period, not only she passionately taught me valuable academic knowledge but she also taught me lots of precious things beside science. I would like to say that having opportunity to be under her supervision has been my highest pleasure. Thanks to her heartfelt advices and supports during my thesis registration and completing report.
Last but not least, a very special thanks goes to Ms. Tran Thi Quynh Dao, Ms. Nguyen Thi Xuan Huong, who has spent countless hours in the lab explaining and instructing me how to carry out the experiments. In addition, I would like to thank all the other officers at Applied Chemistry Laboratory and many third-year students, namely, To VinhTrieu, Nguyen QuanTrinh, Dao Ngoc Phuong Uyen at International University for enthusiastically supporting me during my thesis.
EFFECT OF CHOLESTEROL ON THE PARTITIONING OF
AMITRIPTYLINE INTO LIPID MEMEBRANES
Duyen T.M. Trana, Trieu V. To, Trang T. Nguyenb
aSchool of Biotechnology, International University – Vietnam National University in HCMC
bCorresponding author's email address:
ABSTRACT
In this study, the effect of cholesterol on the partitioning of amitriptyline, a tricyclic
antidepressant, into lipid bilayers composed of 1,2-dioleoyl-sn-glycero-3-
phosphocholine (DOPC), 1-stearoyl-2-oleoyl-sn-glycero-3-phosphocholine (SOPC),
or 1,2-distearoyl-sn-glycero-3-phosphocholine (DSPC) was examined using second
derivative spectrophotometric method. As the results revealed, amitriptyline
preferred to partition into the unsaturated DOPC followed by the mixed chain
(SOPC) and the saturated (DSPC). The presence of 28 mol% cholesterol facilitated
the partitioning of amitriptyline into the saturated and mixed chain lipids (DSPC
and SOPC) but decreased the drug partitioning into the unsaturated lipid (DOPC).
The study showed a significant role of cholesterol on the partitioning of a drug into
the lipid membranes.
Keywords: Amitriptyline, Cholesterol, Liposomes, Second Derivative Spectrophotometer
The therapeutic and toxic effects of drugs are strongly influenced by their lipid
affinity, and the study of drug-lipidmembrane interaction is of importance in drug
absorption, distribution, metabolism and elimination phenomena, as well as in
assessing toxic or therapeutic effects and bioaccumulation. Lipid membranes
contain several hundred types of lipids with different headgroups and acyl chain
compositions whose properties such as charge state and packing density will
influence drug partitioning. The major component of membrane lipids is
glycerophospholipids which are comprised of a polar headgroup and two nonpolar
acyl chains as a tail. The most popular headgroup is phosphatidylcholines (PC)
which are electrically neutral incorporate choline as a headgroup. The two acyl
chains may be saturated, unsaturated or one chain saturated and the other
unsaturated. As stated above, the difference in the unsaturation degree results in
difference in the lipid fluidity and packing density, and thus will affect the
partitioning of the drug to the lipid membrane.
It has been found that one of the most important components of cell membranes
which influences on the cell membranes' activity is cholesterol. Cholesterol is a
modified steroid and plays an essential structural component of cell membranes that
is required to regulate membrane permeability and fluidity by changing their
ordering, available area and formation of domains of composition. At the
molecular level, the most pronounced and easily identified effects of cholesterol are
the so-called ordering and condensing effects on membrane lipids; cholesterol
has a dual nature - it promotes ordering and rigidity of the lipids in the liquid state,
while it's effects are the opposite on the gel state lipids. There are several
studies about drug-lipid membrane interactions and distribution of drug into
lipid membranes depending on the saturation of lipid alkyl chains. In
addition, many studies have revealed the general interaction between cholesterol
and phospholipid bilayer (e.g., cholesterol interacts with all of the lipid in bilayer
membrane, cholesterol-induced fluid membrane domains, complex behavior
phosphocholine/cholesterol). The effect of cholesterol on the structure of lipid
membrane has been studied more clearly (e,g., effect of cholesterol on
phosphatidylcholine bilayer polar region; relationships between hydrophobic
thickness, acyl-chain orientation order of lipid membrane and cholesterol; effect
of cholesterol on molecular order and dynamics in highly polyunsaturated
phospholipid bilayers; importance of double-bond position on interplay of
unsaturated phospholipids and cholesterol in membrane). However,it is still
unclear how cholesterol affects the partition of a drug into lipid membranes.
Because cholesterol fluidizes the lipid membranes if lipids are in the gel-state
whereas the lipid bilayers in the liquid-crystalline state become more ordered with
the presence of cholesterol. Moreover, in the presence of cholesterol, this
involves one assumption that cholesterol occupies more space that prevents the
drug from partitioning into the lipid membrane. Therefore, whether cholesterol
enhances or impedes the partitioning of drugs into the lipids with different
unsaturation degree should be examined.
Amitriptyline is a type of medicine called a tricyclic antidepressant (TCA) which acts
on nerve cells in the brain. When depression occurs, there may be a decreased
amount of serotonin and noradrenaline released from nerve cells in the brain.
Amitriptyline works by preventing serotonin and noradrenaline from being
reabsorbed back into the nerve cells in the brain. This helps prolong the mood
lightening effect of any released noradrenaline and serotonin. In this way,
amitriptyline helps relieve depression. Due to the fact that amitriptyline inhibits
the membrane pump mechanism which responsibles for the uptake of noradrenaline
and serotonin in adrenergic and serotonergic neurons, it has been generally
believed that drug inhibition ability correlates with its mechanism of partition into
lipid membranes.
In this study, the effect of cholesterol on the partitioning of amitriptyline into lipid
membranes was examined. The partitioning of a drug into lipid membranes can be
expressed through a partition coefficient (Kp). Kp is an indicator of the distribution of a drug between lipid and aqueous phases. It is a key parameter in drug design as
the absorption, distribution, metabolism as well as toxicity and therapeutic effects of
a drug involve its passage across lipid membranes. Therefore, the effect of
cholesterol on the lipid membrane partitioning of the drug can be evaluated by the
Kp. The coefficient (Kp) of amitriptyline into the lipid membranes with and without cholesterol (28 mol%) was determined by using stable immobilized unilamellar
liposomes which are model mammalian cell membranes. The partition coefficient of
amitriptyline were examined in 3 lipids which are different in the unsaturation
glycero-3-phosphocholine (SOPC) and 1,2-distearoyl-sn-glycero-3-phosphocholine
(DSPC). All these three lipids are glycerophospholipids, which are comprised of 2
acyl chains and a polar head group. DSPC has two saturated acyl chains while DOPC
is composed of two unsaturated chains. SOPC is the mixed chain lipid with one chain
saturated and the other unsaturated. The chemical structures of DOPC, SOPC and
DSPC were shown in Figure 1. The varying unsaturation degree leads to the
difference in lipid fluidity and packing density, therefore affects the partitioning of
the drug into the lipid membranes.
1) Materials:
phosphocholine (SOPC) and 1,2-distearoyl-sn-glycero-3-phosphocholine (DSPC)
were bought from Avanti polar lipids (USA). Amitriptyline was purchased from
Sigma Aldrich (USA). Cholesterol (99 +% purity, Sigma Chemical Co.). Nanopure
water, distilled from NanopureTM system with impedance of 18.2 MΩ-cm, was used
to prepare all solutes during the experiments. All liquid suspensions were made with
PBS buffer solution (50 mM Na2HPO4.2H2O and 100 mMNaCl (Merck, Germany) at pH 7.4).
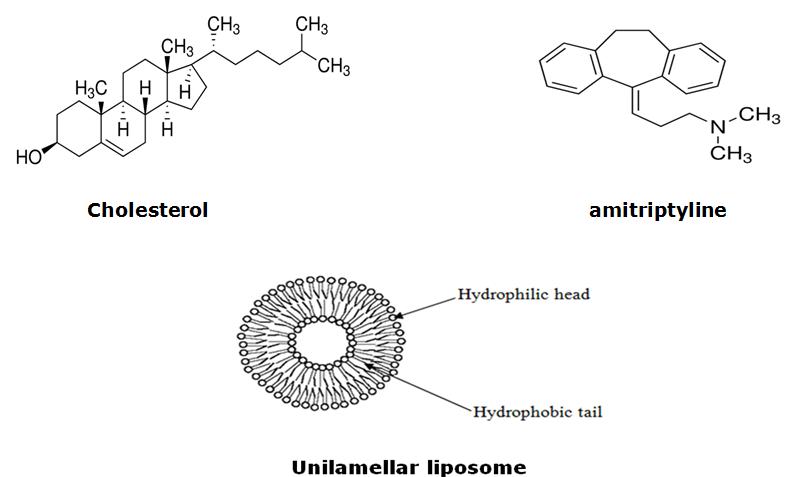
Figure 1:Chemical structures of DOPC, SOPC and DSPC.
Figure 2: Structure of Cholesterol, Amitriptyline (Sigma Aldrich, USA) and
Unilamellar liposome (the liposome structure was taken from FAO Corporate
Document Repository).
2) Liposome and drug/liposome preparation
The pure lipids (DOPC, SOPC and DSPC) and lipids containing 28 mol% cholesterol
were thoroughly mixed in chloroform and then evaporated to dryness under the
stream of nitrogen. The dried lipid film was left under vacuum overnight to remove
all traces of the organic solvent and then stored at -20 oC until used.
In order to prepare liposomes, PBS buffer was added into the dried lipid vial and the
mixture was vortexed to produce multicellular liposomes (MLVs). After that, MLVs
were frozen and thawed by repeating 5 times a cycle of freezing the liposomes in
-20 oC and then thawing in a water bath at 60 oC. Next, the lipid suspensions were
extruded 30 times through polycarbonate filters with a pore size of 0.1 m to
produce unilamellar vesicles (LUVs). During extrusion, the lipid solutions were kept
at the temperature at least 10 oC higher than the phase transition temperature for
each lipid, room temperature for DOPC and SOPC and 65 oC for DSPC.
Sample solutions were prepared by mixing a known volume of drug and suitable
vesicle suspensions. The lipid stock suspension was diluted to prepare a set of
suspension with different lipid concentration (range from 0 to 0.25 mM), in which
the drug concentration was kept constant at 0.0225 mM. A set of blank suspensions
(corresponding reference solutions) were prepared identically but without
amitriptyline for each assay. All suspensions were vortexed for 30 seconds and then
incubated at 37 oC for at least 30 minutes before being measured.
3) UV-Vis measurement
The absorption spectra of all suspensions were collected using Agilent Cary 60 UV-
Vis spectrophotometer with the spectral window from 190nm to 300 nm and
equipped with a constant-temperature cell holder. The absorption spectra of sample
suspensions were obtained by measuring against the corresponding reference
suspension which had the same composition but without amitriptyline. All sample
solutions were measured at 37 oC in a microcell cuvette with a chamber volume of
4) Determination of partition coefficients
Partition coefficients were determined by the derivative spectrophotometry. This
technique is based on the evaluation of the discrete spectral variations presented by
the drug in the presence of increasing lipid concentrations. The liposome/buffer
patition coefficient is defined as the ratio between the concentration of membrane-
bound drug in lipid phase and the concentration of free drug in buffer phase. This
relation can be expressed as:
Where Ct: drug molar concentration
Cm: drug in lipid concentration Cw: drug in aqueous media concentration
[lipid]: lipid molar concentration
[water]: water molar concentration (55.3 M at 37 oC)
According to the Beer-Lambert law, absorbance is directly proportional to
concentration, at a specific wavelength, A = εmCm + εwCw Where εm: drug extinction coefficient in lipid bilayer
εw: drug extinction coefficient in water
Let ∆A is the difference between absorption in the presence and absence of liposomes and related to portioning coefficient (Kp value) by the following equation:
Similar to absorbance, derivative intensity is proportional to the solute concentration. Denoting (dnA/dnλ) by D. From equation (1) and (2), relation between ∆D and Kp could be expressed by the following equation:
Where ∆D is differential absorption of drug in lipid phase at a high concentration of
lipid, that is, when 100 % of drug binds to liposomes, ∆D reaches its maximum
value ∆Dmax, where ∆Dmax = εCt. The values of Kp and ∆Dmax were calculated from the experimental values of molar concentration of lipid and ∆D by applying a non-
linear least-squares method. The derivative spectra were calculated using
OriginPro 9.0 software (OriginLab, Northampton, MA) that involved the Savitzky-
Golay method, in which the second-order polynomial and 17 window points
Employing the derivative spectrophotometry method, light scattering from lipid
vesicles was eliminated before measuring patition coefficient. The Kp values were calculated by fitting experimental data of ∆D and molar concentration
of lipid to equation (3). Applying maximum-peak method for heterogeneous
samples in order to increase reproducibility and signal-to-noise ratio. ∆D values
were collected at λmax of the absorption spectra. Because light scattering as a source of additional noise in absorption measurements, ∆D values used were
obtained atthe wavelength (λmax) where maximum absorbance of amitriptyline was occurred.
RESULTS AND DISCUSSION
1) Absorption spectra
a. Absorption spectra of amitriptyline in pure lipids
The absorption spectra of amitriptyline at a concentration of 0.0225 mM in the
presence of various amounts of lipid vesicles containing DOPC, SOPC and DSPC
were depicted in Figure 3. It is important to point out that the concentration of
amitriptyline used in the study was obeyed Beer's Law for absorption spectra.
The curves (2-8) in Figure 3 were obtained by subtracting the absorption spectrum
of lipid without amitriptyline (the blank) from absorption spectrum of lipid with
amitriptyline recorded at the same lipid concentration. When increasing the lipid
concentration of DOPC, SOPC and DSPC, the maximum absorbance at the
wavelength of 209 nm decreased and the wavelength of the maxima showed
bathochromic shifts – shifts to longer wavelength. Similar shifts in absorption
spectra have been previously observed for chlorpromazine, promazine and
methochlorpromazine. This demonstrated that amitriptyline partitioned into
the LUVs, ie., the environment surrounding amitriptyline became less polar as
amitriptyline partitioned from the aqueous phase to the lipid phase.
Figure 3: Absorption spectra of 0.0225 mM amitriptyline in PBS buffer solution (pH
7.4, 37 oC) containing various amounts of LUVs (DOPC, SOPC, DSPC, respectively).
Lipid vesicles concentrations (mM): (1) 0; (2) 0.025; (3) 0.05; (4) 0.075; (5) 0.1;
(6) 0.15; (7) 0.2; (8) 0.25.
b. Absorption spectra of amitriptyline in lipids containing 28 mol% cholesterol
The absorption spectra of amitriptyline at a concentration of 0.0225 mM in the lipid
vesicles of DOPC, SOPC and DSPC containing 28 mol% cholesterol were shown in
In the presence of 28 mol% cholesterol, the absorption spectra of amitriptyline in
the three lipids (DOPC, SOPC and DSPC) were similar to those in the pure lipids.
The absorbance of amitriptyline in the three DOPC, SOPC and DSPC lipids also
decreased and the wavelengths of the maxima shifted to the right.
The background signals presented by the lipid solutions in the ultraviolet region
which could not be eliminated by zero-order spectra. Applying higher orders of
derivative, particularly, second-order derivative could eliminate baseline shifts, since
scattering by lipid had a negligible effect on the second derivative. Moreover,
second derivative spectrophotometry increased the accuracy of quantification
because spectral details were enhanced and overlapping bands were separated.
DOPC + CHOLESTEROL
SOPC + CHOLESTEROL
DSPC + CHOLESTEROL
Figure 4: Absorption spectra of 0.0225 mM amitriptyline in PBS buffer solution (pH
7.4, 37 oC) containing various amounts of LUVs/Cholesterolvescicles (lipid DOPC,
SOPC, DSPC, respectively). The lipid vesicles concentrations (mM): (1) 0; (2)
0.025; (3) 0.05; (4) 0.075; (5) 0.1; (6) 0.15; (7) 0.2; (8) 0.25.
2) Second derivative spectra of absorption
a. Second derivative spectra of absorptionin the pure lipids
Figure 5: Second derivative spectra of amitriptyline calculated from the absorption
spectra in Figure 3.
The second derivative absorption spectra of amitriptyline in different lipid
concentrations were shown in Figure 5. As can be observed, the interference caused
by the presence of liposomes was completely eliminated with the second derivative.
The second derivative absorbance minima increased in intensity and shifted toward
higher wavelengths.
b. Second derivative spectra of absorbance in the lipids containing 28 mol%
cholesterol
Second derivative absorption spectra of amitriptyline in the lipids containing 28
mol% cholesterol were presented in Figure 6. Similar to what was observed in the
second derivative spectra of amitriptyline in the pure lipids, the second derivative
spectra in the lipids with cholesterol exhibited a bathochromic shift and increased in
the derivative intensity of the minima.
The Kp values were obtained using the data from the second derivative spectra, at a highest wavelength λmax in the absorption spectra (209 nm). The values of Kp were then calculated by fitting experimental data (∆D vs. [lipid]) to Equation (3) at 8
different lipid concentrations. The Kp values obtained were listed in Table 1 for DOPC, SOPC and DSPC and these lipids containing 28 mol% cholesterol.
DOPC + CHOLESTEROL
SOPC + CHOLESTEROL
DSPC + CHOLESTEROL
Figure 6: Second derivative spectra of amitriptyline calculated from the absorption
spectra of Figure 4.
Table 1: Partition coefficients (Kp) of amitriptyline at concentration 0.0225 mM into
the pure lipids DOPC, SOPC and DSPC and these lipids with 28 mol% cholesterol.
Kp values*
0 mol% cholesterol
28 mol% cholesterol
*The values reported were the mean and standard deviation of at least three
independent measurements.
As seen in Table 1, the Kp values of amitriptyline in the lipids DOPC, SOPC, DSPC followed the order: DOPC > SOPC > DSPC. It indicated that the partitioning of
amitriptyline into the unsaturated lipid (DOPC and SOPC) was greater than that of
the saturated lipid (DSPC). Possessing the cis-double bond, DOPC and SOPC
molecules occupy more area ( 75 Å2/DOPC molecule, 65.5 Å2/SOPC molecule
, respectively) than the saturated DSPC molecules ( 50-60 Å2/DSPC
molecule) (see Figure 1). As a result, the more loosely packed DOPC and SOPC
vesicles allow amitriptyline to partition more easily as compared to the more lightly
packed DSPC vesicles. In addition, the experiments was carried out at 37 oC that
was below the main phase transition of DSPC (Tm = 55 oC) and above the main phase transition of SOPC (Tm = 6 oC), DOPC (Tm = -17 oC). Since the physical state of lipid was determined by the transition temperature, DOPC and SOPC were in
liquid – crystalline state, characterized by the high mobility because the acyl chains
are more disordered whereas DSPC was in the solid-gel state with less mobility and
more ordered acyl chains. The weak packability and high fluidity of DOPC and SOPC
facilitated amitriptyline partition more effectively into these lipids relative to DSPC.
This order for the partition of amitriptyline into DOPC, SOPC and DSPC is in
agreement with the previous study, carried out on the partition of haloperidol into
In the presence of 28 mol% cholesterol, the partition coefficient of amitriptyline into
the saturated lipid DSPC and the mixed-chain lipid SOPC increased about 42% and
43%, respectively. In the unsaturated DOPC, however, the partition coefficient of
amitriptyline decreased around 49%. This significant effect of cholesterol on the
partitioning of amitriptyline into the lipid vesicles could be directly related to the
interaction between cholesterol and the lipid vesicles. The ordering effect of
cholesterol has been known to cause gel-state lipids become more disordered (i.e.
fluidizing effect) and liquid-state lipids become more ordered. In the presence of
cholesterol, the more ordered DOPC acyl chains resulted in a more tightly packed
vesicles, reducing amitriptyline partition into the lipid vesicles. In DSPC vesicles,
however, cholesterol fluidizes the gel-state lipid which allowed more amitriptyline
penetrate into. In SOPC vesicles, the ordering effect should be expected since SOPC
stays in the liquid state at 37oC. However, the partition coefficient of amitriptyline in
SOPC did increase in the presence of cholesterol. It could be explained that, for the
mixed-chain phospholipid SOPC containing one saturated chain - the sn1 and one
acyl chain containing a double bond – the sn2 (see Figure 1), a combination of the
ordering effect on the chain sn2 and the fluidizing effect on the chain sn1. The
increase on the partitioning of amitriptyline into SOPC may be caused by the
stronger disordering effect onthe chain sn1 that similar to the fluidity characterof
CONCLUSION
In summary, it was indicated that the weak packability and high liquidity of DOPC
and SOPC allowed amitriptyline partition more effectively as compared to DSPC.
However, in the presence of cholesterol, the stronger fluidizing effect was induced
on saturated DSPC and SOPC while the ordering effect was pronounced on the
unsaturated phospholipids DOPC. As a consequence, cholesterol facilitated the
partitioning of amitriptyline in DSPC and SOPC but inhibited the partitioning of
amitriptyline in DOPC. These results support for the hypothesis, that is cholesterol
has a significant effect on the partitioning of amitriptyline into the lipid membranes
with different unsaturation degrees. In particular, the fluidizing and ordering effect
of cholesterol on the partitioning of drug into the SOPC appears to be an important
and interesting issue, which should be further studied.
REFERENCES
Ma, Q. and A.Y. Lu, Pharmacogenetics, pharmacogenomics, and individualized
medicine. Pharmacol Rev, 2011. 63(2): p. 437-59.
Sharom, F.J., Complex Interplay between the P-Glycoprotein Multidrug Efflux Pump and
the Membrane: Its Role in Modulating Protein Function. Front Oncol, 2014. 4: p. 41.
Ferreri, C., Life—As a Matter of Fat: The Emerging Science of Lipidomics.By Ole G.
Mouritsen. ChemBioChem, 2005. 6(8): p. 1463-1464.
Wydro, P., S. Knapczyk, and M. Łapczyńska, Variations in the Condensing Effect of
Cholesterol on Saturated versus Unsaturated Phosphatidylcholines at Low and High
Sterol Concentration. Langmuir, 2011. 27(9): p. 5433-5444.
Oldfield, E., et al., Spectroscopic studies of specifically deuterium labeled membrane
systems. Nuclear magnetic resonance investigation of the effects of cholesterol in
model systems. Biochemistry, 1978. 17(14): p. 2727-40.
Marsh, D. and I.C.P. Smith, An interacting spin label study of the fluidizing and
condensing effects of cholesterol on lecithin bilayers. Biochimica et Biophysica Acta
(BBA) - Biomembranes, 1973. 298(2): p. 133-144.
Vist, M.R. and J.H. Davis, Phase equilibria of cholesterol/dipalmitoylphosphatidylcholine
mixtures: deuterium nuclear magnetic resonance and differential scanning calorimetry.
Biochemistry, 1990. 29(2): p. 451-464.
Seydel, J.K. and O. Wassermann, NMR-Studies on the molecular basis of drug-induced
phospholipidosis—II. Interaction between several amphiphilic drugs and phospholipids.
Biochemical Pharmacology, 1976. 25(21): p. 2357-2364.
Deo, N., T. Somasundaran, and P. Somasundaran, Solution properties of amitriptyline
and its partitioning into lipid bilayers. Colloids and Surfaces B: Biointerfaces, 2004.
34(3): p. 155-159.
Fisar, Z., K. Fuksova, and M. Velenovska, Binding of imipramine to phospholipid bilayers
using radioligand binding assay. Gen Physiol Biophys, 2004. 23(1): p. 77-99.
Fisar, Z., Interactions between tricyclic antidepressants and phospholipid bilayer
membranes. Gen Physiol Biophys, 2005. 24(2): p. 161-80.
Singer, M.A. and L. Finegold, Cholesterol interacts with all of the lipid in bilayer
membranes. Implications for models. Biophysical Journal. 57(1): p. 153-156.
Marsh, D., Cholesterol-induced fluid membrane domains: A compendium of lipid-raft
ternary phase diagrams. Biochimica et Biophysica Acta (BBA) - Biomembranes, 2009.
1788(10): p. 2114-2123.
Zhao, J., et al., Phase studies of model biomembranes: Complex behavior of
DSPC/DOPC/Cholesterol. Biochimica et Biophysica Acta (BBA) - Biomembranes, 2007.
1768(11): p. 2764-2776.
Pasenkiewicz-Gierula, M., et al., Cholesterol Effects on the Phosphatidylcholine Bilayer
Polar Region: A Molecular Simulation Study. Biophysical Journal, 2000. 78(3): p. 1376-
1389.
Ipsen, J.H., O.G. Mouritsen, and M. Bloom, Relationships between lipid membrane area,
hydrophobic thickness, and acyl-chain orientational order. The effects of cholesterol.
Biophysical Journal, 1990. 57(3): p. 405-412.
Mitchell, D.C. and B.J. Litman, Effect of Cholesterol on Molecular Order and Dynamics in
Highly Polyunsaturated Phospholipid Bilayers. Biophysical Journal, 1998. 75(2): p. 896-
908.
Martinez-Seara, H., et al., Interplay of Unsaturated Phospholipids and Cholesterol in
Membranes: Effect of the Double-Bond Position. Biophysical Journal, 2008. 95(7): p.
3295-3305.
McIntosh, T.J., The effect of cholesterol on the structure of phosphatidylcholine bilayers.
Biochimica et Biophysica Acta (BBA) - Biomembranes, 1978. 513(1): p. 43-58.
Charney, D.S., G.R. Heninger, and D.E. Sternberg, Serotonin function and mechanism of
action of antidepressant treatment: Effects of amitriptyline and desipramine. Archives
of General Psychiatry, 1984. 41(4): p. 359-365.
Fisar, Z., R. Krulik, and D. Beitlova, Liposomes--model membranes to study the binding
of tricyclic antidepressants. Drug Metabol Drug Interact, 1991. 9(3-4): p. 269-81.
Rodrigues, C., et al., Derivative spectrophotometry as a tool for the determination of
drug partition coefficients in water/dimyristoyl-L-alpha-phosphatidylglycerol (DMPG)
liposomes. Biophys Chem, 2001. 94(1-2): p. 97-106.
Kitamura, K., et al., Second derivative spectrophotometric determination of partition
coefficients of chlorpromazine and promazine between lecithin bilayer vesicles and
water. Analytica Chimica Acta, 1995. 304(1): p. 101-106.
Omran, A.A., et al., Determination of partition coefficients of diazepam and flurazepam
between phosphatidylcholine bilayer vesicles and water by second derivative
spectrophotometric method. J Pharm Biomed Anal, 2001. 25(2): p. 319-24.
Savitzky, A. and M.J.E. Golay, Smoothing and Differentiation of Data by Simplified Least
Squares Procedures. Analytical Chemistry, 1964. 36(8): p. 1627-1639.
Griffiths, T.R., et al., Some aspects of the scope and limitations of derivative
spectroscopy. Analytica Chimica Acta, 1982. 143(0): p. 163-176.
Rojas, F.S., C.B. Ojeda, and J.M.C. Pavon, Derivative ultraviolet—visible region
absorption spectrophotometry and its analytical applications. Talanta, 1988. 35(10): p.
753-761.
Kitamura, K., et al., Determination of dissociation constants of sparingly soluble
phenothiazine derivatives by second-derivative spectrophotometry. Analytica Chimica
Acta, 1991. 242(0): p. 131-135.
Luxnat, M. and H.-J. Galla, Partition of chlorpromazine into lipid bilayer membranes: the
effect of membrane structure and composition. Biochimica et Biophysica Acta (BBA) -
Biomembranes, 1986. 856(2): p. 274-282.
Magalhaes, L.M., et al., High-throughput microplate assay for the determination of drug
partition coefficients. Nat. Protocols, 2010. 5(11): p. 1823-1830.
Marsh, D., CRC handbook of lipid bilayers. 1990: CRC Press.
Kucerka, N., M.P. Nieh, and J. Katsaras, Fluid phase lipid areas and bilayer thicknesses
of commonly used phosphatidylcholines as a function of temperature. Biochim Biophys
Acta, 2011. 1808(11): p. 2761-71.
Source: http://csc.hcmiu.edu.vn:8080/dspace/bitstream/handle/123456789/1240/022001752%20-%20Duyen,%20Tran%20Thai%20My.pdf?sequence=1
Phytoprost:
Studien allgemein – Bedeutung in der Prävention von Krebs Cancer Causes Control. 2015 Nov;26(11):1521-50. doi: 10.1007/s10552-015-0659-4. Epub 2015 Sep 9. A systematic review of dietary, nutritional, and physical activity interventions for the prevention of prostate cancer progression and mortality. Hackshaw-McGeagh LE1,2, Perry RE3, Leach VA4,5, Qandil S4, Jeffreys M4, Martin RM3,4, Lane JA3,4.
rahe.in
Ratiram Academy of Higher Education (RAHE) GPAT 2012 ANSWER Q.1. Which of the following respective Phase I and Phase II reactions are the most common drug biotransformation reactions?(A) Oxidation and Glucuronidation(B) Reduction and Acetylation(C) Hydrolysis and Glucuronidation(D) Oxidation and Glutathion conjugationAnswer‐ A