Gaafar_f_rev
STIMULATION AND CONTROL OF E. COLI BY USINGAN EXTREMELY LOW FREQUENCY MAGNETIC FIELD
EL-SAYED A. GAAFAR*, MAGDA S. HANAFY**, EMAN Y. TOHAMY***, MONA H. IBRAHIM** * Biophysics Department, Faculty of Science, Cairo University, Egypt ** Physics Department, Faculty of Science, Zagazig University, Egypt *** Botany Department, Faculty of Science, Zagazig University, Egypt Abstract. The effect of a 50 Hz magnetic field of strength 2 mT on each of the growth characteristics, the antibiotic sensitivity and the ultra structure of E. coli bacteria cells have been studied. Equal volumes of E. coli cells were exposed to the magnetic field for different periods, the two most effective periods, namely, 6 h and 16 h were chosen for all our experimental studies. The results indicated that exposure of the microorganisms to the demonstrated magnetic field caused pronounced changes in the growth characteristic curves, where a suppressive effect was observed on the cell growth and the number of cells at stationary phase markedly decreased after exposure period of 6 h but there was a slight increase in the growth rate after exposure period of 16 h with increase in the number of cells. Further, changes in the antibiotic sensitivity was observed after exposure period of 6 h since E. coli cells became more sensitive to certain antibiotics such as amoxicillin, nalidixic acid and erythromycin as revealed in the increase in their zone diameters while, after a 16 h exposure period, it became more resistant to the same antibiotics. Furthermore, the results of the ultra structure showed that while exposure period 6 h decreased the cell length, the exposure period 16 h elongated the cell length with decreasing the thickness of the cell wall beside the disappearance of the majority of cytoplasmic components. Keywords: electromagnetic field, E. coli, growth rate, antibiotic sensitivity, ultra structure. During the past few decades, due to the increasing consumption of electric energy in industry, medicine, research, communication systems and household electric appliances, the level of exposure of biological systems to electromagnetic fields has grown by orders of magnitude over a wide frequency range extending from 0 to 100 GHz. For example, hair dryers, electric shavers and electric hand tools may expose the user to magnetic fields of several times above the background. Received July 2006; in final form September 2006. ROMANIAN J. BIOPHYS., Vol. 16, No. 4, P. 283–296, BUCHAREST, 2006 El-Sayed A. Gaafar et al. The extremely low-frequency (ELF) electromagnetic field (EMF) exists in all occupational and residential environments. Some scientists allege that exposure to magnetic fields generated by power delivery systems is responsible for certain diseases; therefore, it is both appropriate and important to evaluate the possible effects of man-made electromagnetic field on living organisms. Fadel et al. [7] reported that the main damaging role of the 50 Hz magnetic fields might be on the cellular membrane that strongly affects, not only the cellular physiological functions, but also the cell-to-cell communications. Ma Haile et al. [15] studied the effect of pulsed magnetic field intensity and pulse number (PMF) on bactericidal property of PMF in sterilization of fresh watermelon juice. Their results showed that the overall bactericidal effect was strengthened as the magnetic field intensity and pulse number increased with the best effect observed when the magnetic field intensity was 2.53 T and pulse number was 20. Piatti et al. [23] found that the exposure of Serratia marcescens to a static magnetic field of 80 ± 20 Gauss resulted in the inhibition of S. marcescens growth. Dacosta [5] and Barnickel [3] used new non-destructive decontamination technique to reduce the bacteria in milk, orange juice and also in cheese. Pulsed electric field, pulsed magnetic field and pulsed light were used. Fojt et al. [8] found that E. coli, Leclercia adecarboxylata and Staphyloccus aureus viability was affected with the magnetic field (10 mT, f = 50 Hz) they also found that the decrease of the colony forming units (CFU) starts immediately after the magnetic field was switched on. Mei et al. [16] studied the inactivation of microorganisms by a pulsed magnetic field. It was reported that the application of electromagnetic pulses evidently causes a lethal effect on E. coli cells suspended in buffer solution. Shengying et al. [31] studied the non-thermal sterilization by using the self- designed generator of magnetic field. The results showed that the magnetic flux density, which had the greatest effect on E. coli, was 1 T. The greatest destruction rate of E. coli was 78% under 8 hours of magnetic field (1 T) treatment. Also, Mohamed et al. [18] reported that exposure of the microorganism S. typhi to the magnetic field (10, 20 G for a period of 2 hours) caused pronounced changes in the growth characteristics and the number of cells at the stationary phase increased. Electromagnetic fields are also used in therapy to enhance the transdermal drug delivery [21]; in certain dairy industry to manipulate growth characteristics of yogurt culture, where the change in culture metabolism rather than an elevation in the overall bacterial population, was induced by a 60 Hz, 4.3 G EMF [17]; in soil studies and inactivation of indicator bacteria of cattle slurry by exposure of 400 – 700 g for 60 seconds to magnetic field (380 V, 50 Hz) [10]; in some food Stimulation and control of E. coli by magnetic field preservation to control certain pathogenic bacteria such as Salmonella and E. coli contaminated meat samples [4]; in agriculture to improve soil fertility by increasing the nodulation process by exposure of Rhizobium sp. to a low strength (5×10–3 T) EMF before inoculation to mycorrhizal chick-pea seedlings [2]. Finally, EMF has been used either to inhibit or to stimulate the growth rate of microorganisms under appropriate conditions [10]. This work is concerned with the study of the biological effect of magnetic fields, as a component of the non-ionizing radiations, on a unicellular system. Pathogenic microorganisms, especially Escherichia coli, are chosen to be our experimental model for many reasons; it is widely distributed in the environment such as soil, water and air. E. coli is a member of the normal intestinal flora of humans. It causes several diseases such as urinary tract infection, wound infection, traveller's diarrhea. It reaches blood stream and causes sepsis and meningitis [25]. E. coli are rapidly growing, Gram-negative, rod-shaped cells measuring approximately 0.5×2 µm length [20]. In the light of the pathogenic effects of these bacteria, we aim, here, to study the effect of different exposure periods to a 20 G, 50 Hz magnetic field on the cell activity with the aim to control the activity through the exposure period. Moreover, we intend to take two exposure periods for investigating the effect of such a magnetic field on the growth rate, the antibiotic sensitivity and the ultrastructure of the exposed cells. MATERIAL AND METHOD
BACTERIAL STRAIN Escherichia coli ATCC ≠ 25992 was cultivated over night on Nutrient broth at 37 °C; each ml of bacterial suspension contained 13 × 103 CFU/ml. To study the bacterial growth, a standard survival curve was plotted between the absorbance of volume A (unexposed cells) at 600 nm and the concentration of cells (number of cells / mL). For cell counting the plate count technique was used [28]. Appropriate dilutions of the bacterial cells were used to inoculate nutrient agar plates. Inoculated plates then incubated at 35 °C for 24 h by counting the number of colonies developed after incubation and multiplying it with the dilution factor the number of cells in the initial population is determined. MAGNETIC FIELD EXPOSURE FACILITY Bacteria volumes were exposed to a homogeneous magnetic field generated by a solenoid consisting of 320 turns from electrically insulated 2 mm copper wire El-Sayed A. Gaafar et al. wound in a homogeneous way around a copper cylinder 1.5 mm thick, 40 cm diameter and 25 cm length. The cylinder wall was earthed to eliminate the electric field components effects. The magnetic field generator was temperature controlled during the exposure period by using a water pump as shown in Fig 1. The temperature during the exposure period was 37 °C. The tubes of the exposed bacteria were put in the middle of the coil by using supports inside it to get a homogeneous and higher magnetic fields strength. The ends of coil are connected to variac fed from the mains (220 V, 50 Hz). The field strength was 20 G and adjusted by changing the voltage through the coil. Fig. 1. Magnetic field exposure facility. GROWTH CHARACTERISTICS Ten volumes from the strain were incubated for 18 h and then exposed to different exposure periods. The first volume was exposed for two hours; the second volume was exposed for four hours; the third volume was exposed for six hours and so on until 20 h. For each exposure volume, there was a corresponding control volume. Through measuring the absorbance of every volume, the two volumes exposed to the two periods, 6 h and 16 h, were chosen for additional investigations concerning the growth rate, the antibiotic sensitivity and the ultra structure of the cells due to their high effects. Stimulation and control of E. coli by magnetic field Four volumes were used in this study: A, B, C, and D. Volume A is the control of volume B exposed to 6 h magnetic field, volume C is the control of volume D exposed to a 16 h magnetic field. The growth rates of all volumes (A, B, C and D) were determined through measuring the absorbance of the viable cells after 2, 4, 6 until 24 h. The absorbance of the volumes was measured and then plotted as a function of time. Spectronic 20+ Series Spectrophotometer (USA) was used for this purpose. ANTIBIOTIC SUSCEPTIBILITY TEST IN VITRO The isolated E. coli cells were tested for their in vitro susceptibility to various antibiotics such as erythromycin 10 µg, chloramphenicol 30 µg, cefodoxil 30 µg, nalidixic acid 30 µg, garamycin 10 µg, and amoxicillin 25 µg by disk diffusion test according to Baker et al. [1]. The antibiotics used in this study were chosen to be with different modes of action. The diameters of the inhibition or stimulation zone of the volumes A, B, C, and D were measured after 24 h from the exposure process. ULTRA STRUCTURE OF BACTERIA CELLS Volumes A, B, C and D were prepared for the transmission electron microscope by the method recommended by Philipe [22] to define the changes in the morphological structure of E. coli cells. RESULTS AND DISCUSSION
The results obtained in this work concern the induced changes in the structure and the characteristic behavior of E. coli resulting from the exposure to the demonstrated magnetic field. These results may be of a great importance for evaluating the benefits as well as the hazards of the exposure to the low frequency low-level magnetic field. Also the importance of this work lays in the fact that E. coli as a microorganism is a unit cell behaving as a complete alive biological system. Fig. 2 shows the variation of the number of microorganisms in CFU/ml as a function of the sample absorbance measured at 600 nm. The results show the linear dependence of the absorbance on the number of microorganisms in CFU/ml. By using this relation we can calculate the number of the microorganisms/ml (C) from El-Sayed A. Gaafar et al. the measured value of its absorbance (A). The relation can easily express the linear dependence: . ×10 A Fig. 2. Survival curve between log number of bacteria cells/ml and the absorbance at 600 nm. GROWTH CHARACTERISTICS CURVE Fig. 3 shows the change in the absorbance of bacterial strain as a function of the time of exposure to the magnetic field. It is clear from this figure that the exposure periods 2, 4, 6, 8, 10 and 12 hrs decreased the absorbance and, in accordance with equation (1), indicate a decrease in the cells number and consequently an inhibition case for the bacteria. However, at the exposure periods of 14, 16, 18 and 20 h the increased absorbance relative to their control indicates an increase in the cells number and a stimulation case. These results are in a good agreement with Mohamed et al. [18] where the number of cells of S. typhi microorganism exposed to 20 G magnetic fields for 2 hours increased relative to those unexposed. Also, Jaffe [10] reported that the electromagnetic field was used either to inhibit or to stimulate the growth of the microorganism under appropriate conditions. Stimulation and control of E. coli by magnetic field For this reason we used the exposure period of 6 h (volume B) as an inhibition case where the number of cells was 108 and became 107 cells/ml also the exposure period of 16 hours (volume D) as stimulation case where the number of cells was 3.5×102 and became 3.5×104 cells/ml. Moreover, we intend to take the two exposure periods for investigating the effect of the magnetic field (20 G, 50 Hz) on the growth rate, the antibiotic sensitivity and the ultrastructure of the exposed cells. Fig. 3. Absorbance at 600 nm of E. coli cells at a different exposure periods. EFFECT OF MAGNETIC FIELD ON BACTERIAL GROWTH RATE Fig. 4 shows the growth rate of volumes A and B. It is clear from the figure that there is a decrease in the growth rate of the E. coli cells exposed to 6 h relative to its unexposed ones. Fig. 5 explains the growth rate of volumes C and D. It is clear from the figure that there was a slight increase in the growth rate of the exposed E. coli cells relative to its unexposed. The results in Figs. 4–5 and the calculated data from these curves in Table 1 indicate considerable changes in the growth curve characteristics for the two exposure periods 6 and 16 hrs. For the exposure period of 6 h (volume B) the maximum growth occurred at 16 h while for the unexposed cells at 18 h; also, the El-Sayed A. Gaafar et al. maximum number of microorganisms decreased to be 2×107 cells/ml as compared with the unexposed cells 8×109 cell/ml. These results are in a good agreement with M. Li et al. [12] who used magnetic field for 4 h (0.2 kWh/m2) to decrease the survivability of E. coli to reach 0.01%. However, for the exposure period of 16 h (volume D) the maximum growth occurred at 14 h with increasing the maximum number of the microorganism to be 2×1010 cells/ml as shown in the table. Moreover, from these results one sees how the period of the active growth (log phase) decreased for the two volumes B and D, which became 12 and 10 h, respectively, while it was for the unexposed cell 14 h and also the lag phase was short. In spite of these facts, the exposure period of 16 h increased the cell division rate in a good agreement with Nascimento et al [19] who concluded that the electromagnetic field (8 h, 5 G, 60 Hz) had a positive effect in the consume of glucose and growth of E. coli. They attributed the increase in the growth to the shortening of lag phase and excitement of log phase. Fig. 4. Growth rate of E. coli before (unexposed) and after exposure period of 6 h. Potenza [24] suggested that exposing E. coli cultures to 300 mT static magnetic field may stimulate transposition activity. Stimulation and control of E. coli by magnetic field Fig. 5. Growth rate of E. coli before (unexposed) and after (exposed) exposure period of 16 h. Growth characterization of E. coli before and after exposing to the magnetic field Stationary phase No. of cells/ml at stationary phase The inhibitory effect of EMF after an exposure period of 6 h on the growth of bacteria may be due to the interaction between electric charges induced by EMF and that of the cytoplasmic membrane resulting in partial abolishment of electric potential of the cytoplasmic membrane with a subsequent decrease in the macromolecular biosynthesis. Also EMF may cause damage of bacterial DNA and inhibition of its replication [9, 14, 27]. Since the present data proved the cellular membrane of the microorganism had been affected by the external magnetic field, then one expects a disturbance in their metabolic activity and, consequently, a change in their cell division in a good agreement with Mohammed et al. [18] who reported that exposing S. tyhi to a 20 G magnetic field increased their cell division and cell number. El-Sayed A. Gaafar et al. EFFECT OF MAGNETIC FIELD ON BACTERIA ANTIBIOTIC SENSITIVITY Table 2 and Fig. 6 illustrate an increase in the sensitivity of volume B to the antibiotics used especially erythromycin, nalidixic acid and amoxicillin as revealed in the increase of the zone diameter of the microorganism of that volume. These results indicated that the exposed cells became more resistant to the field. Fig. 6 and Table 2 illustrate a decrease in the sensitivity of the exposed cells, volume D, where the diameter of its zones decreased for some antibiotics such as chloramphenicol, amoxicillin and nalidixic acid. These results indicate that the viability of cells exposed to 16 h increased as compared with the unexposed cells (stimulation case). All these results indicate that there are effects of the used electromagnetic field to drug mode of action on bacterial cell through inhibition of, cell wall synthesis, protein synthesis, nucleic acids, essential enzymes and change in membrane permeability [11]. Moreover, Stansell et al. [29] stated that exposing the bacteria to medium strength magnetic field could significantly alter antibiotic sensitivity. He, also, found that exposing E. coli to the magnetic fields considerably increased antibiotic resistance. The antibiotic test of exposed and unexposed E. coli Inhibition zone diameter in cm Unexposed Exposed (6 h) Chloramphenicol Inhibition of protein synthesis Inhibition of bacterial enzymes, prevents formation of PAB (para amino benzoic Inhibition of respiration of Inhibition of cell wall synthesis of bacteria Inhibition of bacterial protein synthesis and act on ribosome Inhibition of protein synthesis and binding of ribosome
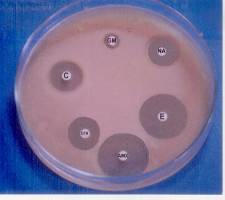
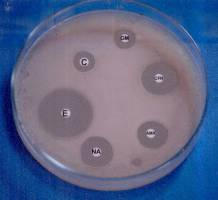
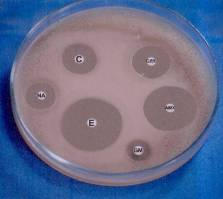
Stimulation and control of E. coli by magnetic field
Fig. 6. The antibiotic zones for the unexposed and exposed bacteria (6 h and 16 h, respectively).
EFFECT OF MAGNETIC FIELD ON ULTRA STRUCTURE OF BACTERIA CELLS
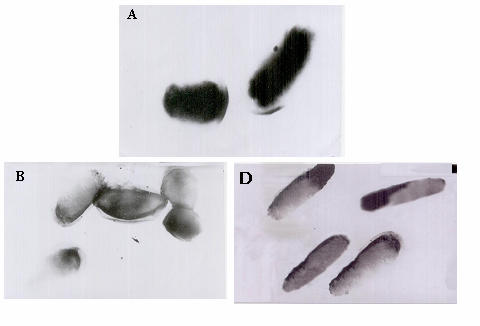
Fig. 7 shows the ultra structure of E. coli cells for the three volumes, A, B and D.
The figure illustrates a complete lyses of the cell wall without destruction of cytoplasmic membrane, granular ribosomal distribution and no vacuoles appear in the cytoplasm for volume B. But for volume D, an elongation of the cells was observed with an increase in the wall thickness of cell and the majority of the cytoplasmic component disappeared.
Strasak et al. [30] found that magnetic field effects depend on the cells shape. To get better understanding of the interaction mechanism of the magnetic
field with biological systems an understanding of the bioelectrical signals resulting from the biological system during metabolic activity is required.
El-Sayed A. Gaafar et al.
Fig. 7. Ultrastructure of the unexposed and exposed bacteria cells (magnification 20×103).
Mohamed et al. [18] reported that the bioelectrical signals from the
microorganism normally were carried out through bending of their cellular membranes, which generate an electric impulse through a phenomenon known as flexoelectricity. The amplitude and the frequency of these impulses depend on the amount and frequency of bending. These impulses travel through the medium separating the microorganisms and were received by the signal receptors at the surface and that impeded in the cell membrane. Therefore the flexibility of the membrane is the most important parameters for generation of these signals. Also mentioned is that the biomagnetic field from the biological system associating to the bioelectrical signals from the membrane of the cells through its metabolic function is very weak, in nanogauss range (20×10–8 G). When the biological systems exposed to an external magnetic field whose strength is very large relative to the biomagnetic field of the cells a disturbance in their metabolic function will be expected and lead to death of the cells or to the increase of their cell division [7, 26]. Del-Re et al. [6] found that E. coli bacteria that had been exposed for a long time to a 50 Hz, low intensity (0.1–1 mT) magnetic field gave colonies with significantly lower transposition activity compared to sham-exposed bacteria. Such reduction in transposition activity was positively correlated to the intensity of the
Stimulation and control of E. coli by magnetic field
EMF, in a dose effect manner also Zhang et al. [32] concluded that strong SMF induce mutations through elevated production of intracellular super oxide radicals in E. coli cells.
From the present data it is easily deduced that the cellular membrane of the
microorganism had been affected by the external magnetic field in a good agreement with Fadel et al. [7]. Then we can expect the disturbance of cell division and hence, a change in the number of the cells per ml or the measured change in the membrane sensitivity to antibiotic demonstrated also the change in the internal structure of the cells.
CONCLUSION
From this work, it is concluded that the electromagnetic field (20 G) affected
considerably the virulence of E. coil cells. 6 h exposure time was found to cause an inhibition case whereas 16 h exposure time enhanced the virulence.
BAKER, F.J., M.R. BREACH, Medical microbiological techniques, Butterworths, 1980.
BAJWA, R., S. MAHMOD, A. ASHFAG, G. NASIM, Effect of electromagnetism on
nodulation, vesicular arbuscular mycorrhizal infection and top growth of chickpea. I. Response
of electromagnetized rhizobium. Pakistan, Journal of Phytopathology, 1995, 7(1), 76–77.
BARNICKEL, M., Prospects for alternative methods of processing, Part 1 DMZ,
Lebensmittelindustrie und Milchwirtschaft, 1998, 119(2), 64–72.
BOROVKOV, M.F., M.M. ALFA, A.M. TSVETKOVA, Influence of microwave
electromagnetic field on meat contaminated with Salmonella and E. coli, Aktual'nye Voprosy Infekksionnykh Invazionnyhk Boleznei Zhivonykh, 1994, 130–132.
DACOSTA, Y., Pulsed electric field – A promising technique, Revue laitiere Francaise, 1997,
575, 32–34.
DEL-RE, B., F. GAROIA, P. MESIRCA, C. AGOSTINI, F. BERSANI, G. GIORGI, Extremely
low frequency magnetic fields affect transposition activity in Escherichia coli, Radiat.
Environ. Biophys., 2003, 42(2), 113–118.
FADEL, M.A., S.M. WAEL, R.M. MOSTAFA, Effect of 50 Hz, 0.2 mT magnetic fields on
RBC properties and heart functions of albino rats, Bioelectromagnetics, 2003, 24, 535–545.
FOJT, L., L. STRASAK, V. VETTERL, J. SMARDA, Comparison of the low-frequency
magnetic field effects on bacteria Escherichia coli, Leclercia adecarboxylata and
Staphylococcus aureus, Bioelectrochemistry, 2004, 63, 337–341.
HYSON, K.P., The biological effects of electromagnetic fields (Bioassays), PHD, Montana-
State-University, 1995.
10. JAFFE, L., in: Biological structures and coupled flows, A. Optlaka, M. Balaban, Academic
Press, New York, 1983.
11. KLACHKOVA, Y.F., M.E. KVITKINA, Disinfecting cattle slurry by treatment with
electromagnetic radiation, Problemy Veterinarnoi Sanitarii I., Ekologii, 1993, 2, 14–18.
12. Li, M, J.H. QU, Y.Z. PENG, Sterilization of Escherichia coli cells by the application of pulsed
magnetic field, J. Environ. Sci., 2004, 16(2), 348–352
13. LORIAN, V., Antibiotics in Laboratory Medicine, Williams and Wilkins, USA, 1986. 14. LOURENCINI-DA-SILVA, R., F. ALBANO, L.R. LOPES-DOS-SANTOS, J.R. TAVARE
A.D., I. FELZENSZWALB, The effect of electromagnetic field exposure on the formation of
DNA lesions, Redox Rep., 2005, 5(5), 299–301.
El-Sayed A. Gaafar et al.
15. MA, H., Y. DENG, J. CHU, H. MA, Y. DENG, Y. CHU, Sterilization of watermelon juice with
high voltage pulse magnetic field and its mechanism analysis, Transactions of the Chinese
Society of Agricultural Engineering, 2003, 19(2), 163–166.
16. MEI, L., Q. JIU-HUI, P. YONG-ZHERI, Sterilization of Escherichia coli cells by the application
of pulsed magnetic field, Journal of Environmental Sciences, 2004, 16, 349–352.
17. MICHAEL, B.D., Manipulation of growth characteristics of yogurt culture by application of an
electromagnetic field, MSC, University of Manitoba, Canada, 1992.
18. MOHAMED, A.A., F.M. ALI, E.A. GAAFAR, H.R. MAGDA, Effects of magnetic field on the
biophysical, biochemical properties and biological activity of Salmonella typhi., Master thesis submitted for Biophysics department, Faculty of science, Cairo University , Egypt, 1997.
19. NASCIMENTO, F., G.J. BOTURA, R.P. MOTA, Glucose consume and growth of E. coli under
electromagnetic field, Rev. Inst. Med. Trop. Sao Paulo, 2003, 45(2), 65–67.
20. NEIDHARDT, F.C., J.L. INGRAHAM, M. SHAECHTER, Physiology of the bacterial cell: A
molecular approach, Sunderland, MA: Sinuer Associates, Inc., USA, 1990.
21. PARASRAMPURIA, D., J. PARASRAMPURIA, Percutaneous delivery of proteins and
peptides using ionotophoretic techniques, J. Clin. Pharmacol. Ther., 1991, 16(7), 17–20.
22. PHILIPPE, G., Manual Methods for General Bacteriology, G. Philippe, ed. American Society
for Microbiology, USA, 1981.
23. PIATTI, E., M.C. ALBERTINI, W. BAFFONE, D. FRATERNALE, B. CITTERIO, M.P.
PIACENTINI, M. DACHA, F. VETRANO, A. ACCORSI, Antibacterial effect of a magnetic
field on Serratia marcescens and related virulence to Hordeum vulgare and Rubs fruticosus
callus cells, Comparative Biochemistry and Physiology, B, Biochemistry and Molecular
Biology, 2002, 132(2), 359–365.
24. POTENZA, L., L. UBALDI, R. DE-CANCTIS, R. DE-BELLIS, L. CUCCHIARINI, D.
CACHA, Effects of a static magnetic field on cell growth and gene expression in Escherichia
coli, Mutat. Res., 2004, 561(1–2), 53–62.
25. PRATS, G., F. NAVARRO, B. MIRELIS, D. DALMAU, Escherichia coli serotype 015:k52:H1
as an uropathogenic clone, J. of Clinical Microbiology, 2000, 38(1), 201–209.
26. SHIN-ICHIRO, H., I. YOSHIMASA, O. KAZUMASA, A. TAKASHI, S. MAKOTO, Change in
broth culture is Associated with significant suppression of E. coli death under high magnetic
field, Bioelectrochemistry, 2002, 57, 139–144.
27. SHUN, H.L., C. KING-CHUEN, Magnetic field exposure induces DNA degradation,
Biochemical and Biophysical research communications, 2001 280, 1385–1388.
28. STAINER, R.Y., J.L. INGRAHAM, M.L. WHEELIS, P.R. PAINTER, General Microbiology,
Macmillan Education, USA, 1986.
29. STANSELL, M.J., W.D. WINTERS, R.H. DOE, B.K. DART, Increased antibiotic resistance of
E. coil exposed to static magnetic field, Bioelectromagnetics, 2001, 22(2), 129–137.
30. STRASAK, L., V. VETTERL, L. FOJT, Effect of 50 Hz magnetic fields on the viability of
different bacteria, Electromagnetic Biology and Medicine, 2005, 24(3), 293–300.
31. YE, S., W. HUANG, M. HE, Q. HE, L. YANG, S. YE, W. HUANG, M. HE, Q. HE, L. YANG,
Preliminary study on technology of magnetic field non-thermal sterilization, Transactions of
the Chinese Society of Agricultural Engineering, 2003, 19(5), 156–160.
32. ZHANG, Q.M., M. TOKIWA, T. DOI, T. NAKAHARA, P.W. CHANG, N. NAKAMURA, M.
HORI, I.J. MIYAKOSH, S. YONEI, Strong static magnetic field and the induction of
mutations through elevated production of reactive oxygen species in Escherichia coli soxR,
Int. J. Radiat. Biol., 2003, 79(4), 281–286.
Source: http://www.biophysicsnet.ro/rjb/articles/165/eagaa.pdf
Pii: s0378-8741(99)00085-9
Journal of Ethnopharmacology 68 (1999) 3 – 37 Aloe vera leaf gel: a review update T. Reynolds a,*, A.C. Dweck b a Jodrell Laboratory, Royal Botanic Gardens, Kew, Richmond, Surrey, UK b Dweck Data, 8 Merrifield Road, Ford, Salisbury, Wiltshire, UK Received 20 April 1999; accepted 20 May 1999
Logo 28_issue 4_en.pdf
ISSUE 4 2012 CAMLOG Partner Magazine THE NEW CAMLOG APP FOR THE TITLE STORY Mobile end devices have long since found their way into the dental practice and for good reason. They inspire through technology and design and provide effective support in many work situations on demand. But there is more to it than just the end device: The right app